Advances in Fenton and Fenton Based Oxidation Processes for Industrial Effluent Contaminants Control-A Review-Juniper Publishers
Juniper Publishers- Open Access Journal of
Environmental Sciences & Natural Resources
Advances in Fenton and Fenton Based Oxidation Processes for Industrial Effluent Contaminants Control-A Review
Authored by Sina Matavos-Aramyan
Abstract
This paper reviews the main advances in Fenton and Fenton-based systems as oxidation processes for environmental pollution abatement in three parts. The increase in the disposal of refractory organics demands for newer technologies for the complete mineralization of these wastewaters. Fenton reaction is an advanced oxidation process which has gained wide spread acceptance for higher removal efficiency of recalcitrant organic contaminants under wide range of operational conditions. However, the requirement of strict acidic conditions to prevent iron precipitation still remains the bottleneck for iron-based Fenton and Fenton-based processes.
The first part of this paper presents a literature review of the various Fenton reagent reactions which constitute the overall kinetic scheme with all possible side reactions. The second part presents a general review on Fenton and Fenton-based processes developed to degrade organic pollutants. Also fundamentals and main applications of typical methods such as Fenton, electro-Fenton, photo-Fenton, sono- photo-Fenton, sono-electro-Fenton and photo-electro-Fenton are discussed. The last part of this paper presents a review of alternative noniron Fenton catalysts and their reactivity towards hydrogen peroxide activation. Elements with multiple redox states, all directly decompose hydrogen peroxide into hydroxyl radicals through conventional Fenton-like pathways. This part of the present paper also highlights limitations influencing their environmental applications.
Keywords: Fenton's reagent; Wastewater treatment; Advanced oxidation processes; Hydrogen peroxide; Transition metal substituted iron oxide
Abbreviations: COD: Chemical Oxygen Demand; BOD: Biological Oxygen Demand; CMCD: Carboxymethyl b-Cyclodextrin; IBP: Ibuprofen; AMX: Amoxicillin; DOC: Dissolved Organic Carbon; PRE: Petroleum Refinery Effluent; ROS: Reactive Oxygen Species; MTBE: Methyl Tert-Butyl Ether; TAME: Tert-Amyl Methyl Ether
Introduction
The global concern about alternative water reuse techniques are increasing continuously as heavy industries such as petrochemicals and agricultures grows bigger each year. Those organic pollutants present in wastewaters which are not treatable by conventional treatment methods, are considered to be treated by stronger and more modern techniques. The presence of these organic compounds in water poses serious threat to public health since most of them are toxic, endocrine disrupting, mutagenic or potentially carcinogenic to humans, animals and aquatic life in general. Wastewaters containing these compounds are known to be high in chemical oxygen demand (COD) and low in biological oxygen demand (BOD). These non- biodegradable molecules enter the environment predominantly through industrial activities.
Traditional wastewater management methods using biological microorganisms (biodegradation) and/or physicochemical processes (flocculation, chlorination, ozonation and misc.), subsequently followed by filtration and adsorption-based separations are able to treat a majority of anthropogenically- polluted water sources. However, no single method described above is efficient enough to produce water with legally- and practically-acceptable levels of refractory toxic chemicals. Physico-chemical techniques like flocculation, adsorption and reverse osmosis require additional post-treatments to prevent secondary disposal and contamination [1]. Wet air oxidation of effluents with >100 g/L of chemical oxygen demand (COD) generates high concentrations of toxic byproducts like dioxins and furans [2]. Direct oxidation processes are widely used to degrade bio-refractory substances. High degradation efficiencies are possible with direct oxidation techniques. However, pollution load, process limitations and operating conditions are the key factors to be considered during the selection of most appropriate oxidation process for a particular compound degradation. Apart from high degradation efficiency, direct oxidation processes demand specified operating conditions to degrade the target compounds and this will increase the operation cost of the process [3-7].
Advanced Oxidation Processes are those techniques which have the capabilities of utilizing the high reactivity of hydroxyl radicals in driving oxidation processes. The different types of AOPs are considered for wastewater pollution abatement [8]. The hydroxyl radicals are extraordinarily reactive species, which attack the most part of organic molecules with rate constants usually in the order of 106-109M'1 s'1 [9]. Hydroxyl radical is the second strongest oxidant preceded by the fluorine, and it reacts 106-1012 times faster than ozone depending on the substrate to be degraded [3,10]. Advanced oxidation processes can be classified either as homogeneous or heterogeneous. Homogeneous processes can be further subdivided into processes that use energy and processes that do not use energy. A goal of the wastewater purification by means of AOP methods is the mineralization of the contaminants to carbon dioxide, water and inorganic or, at least, at their transformation into harmless products. Obviously the methods based on chemical destruction, when properly developed, give complete solution to the problem of pollutant abatement differently from those in which only a phase separation is realized with the consequent problem of the final disposal [3] (Table 1).
Fenton Chemistry
The oxidation processes utilizing activation of H2O2 by iron salts are referred to as Fenton’s reagent. This reaction allows the generation of hydroxyl radicals as shown in reaction (1) [11-13]:
Fe2++H2 O2→Fe3++OH-+OH. (Chain initiation) k1=70M-1s-1 [14] ------(1)
The generation of the radicals involves a complex reaction sequence in an aqueous solution:
OH.+Fe2+→OH+Fe3+(chain termination)k2=3.2 х108M-1s-1 [14] ------ (2)
Fe3+ produced can react with H2O2 and hydroperoxyl radical in the so-called Fenton-like reaction, which leads to regenerating Fe2+ (reactions (3) and (5)). Fe2+ regeneration is also possible by reacting with organic radical intermediates (reaction (7)) [10,11]:
Fe3 ++H2O2→Fe2++ HO2+.H+ k3 = 0.001-0.01 M-1s-1 [15]----(3)
HO2+.Fe2+→HO2+Fe3 k4 = 1.3х106M-1s-1 at pH=3 [16] --- (4)
Fe3 ++ HO2.→Fe2++O2+H+ k5 =1.2х106 M-1s-1at pH=3 [16] ---- (5)
OH.+H2O2→H2O+HO2. k6=3.3х107 M-1s-1 [17] ----- (6)
Fe3++ R. →Fe2++ R+ ----- (7)
If the concentrations of reactants are not limiting, the organics can be completely detoxified by full conversion to CO2, water and in the case of substituted organics, inorganic salts if the treatment is continued.
Reaction (1) which is the overall Fenton chemistry is simplified [18] by accounting for the dissociation water:
2Fe2++ H2O2+2H+ → 2Fe3 ++2H2O -----(8)
This equation indicates the need for an acidic environment to produce the maximum amount of hydroxyl radicals. Previous Fenton studies have shown that acidic pH levels near 3 are usually optimum for Fenton oxidations [19]. At low pH levels and in the presence of organic substrates, hydroxyl radicals can abstract a hydrogen atom, initiating a radical chain oxidation [11,18-20].
RH+ OH. → H2O+ R. (Chain propagation) (9)
R.+ H2O2 →ROH+OH.----- (10)
R.+ O2→ROO.---- (11)
The sequence of reactions (1), (2), (9) and (7) constitute the present accepted scheme for the Fenton's reagent chain. The foregoing analysis indicates that hydrogen peroxide may act both as radical generator as in reaction (1) and as scavenger as in reaction (6) [11].
In the absence or presence of any organic molecule to be oxidized, the decomposition of hydrogen peroxide to molecular oxygen and water occurs according to reaction (10). This reaction leads to exploitation of bulk oxidant and thus an unnecessary increase on treatment cost [3,21]
s2H2O2 → O2+2H2O -----(12)
Fenton oxidation has been extensively applied to the treatment of diverse wastewaters from olive oil industries [22], textile industries [23], paper pulp factories [24], cork processing facilities [25] and winery industries [26], as well as effluents from refinery and fuel terminals [27], sludge waste [28], landfill leachate [29,30] and contaminated soils [31-33].
The main reasons for the huge popularity and widespread applicability of Fenton oxidation processes are [1]:
1. The high efficiency of mineralization enables the transformation of organic pollutants into non-toxic CO2,
2. Owing to the rapid reaction between iron and H2O2, the activation of H2O2 and the subsequent generation of hydroxyl radicals are completed in the shortest reaction time among all other AOPs [1,34],
3. Oxidizing radicals are generated at ambient pressure and temperature, which avoids the requirement of complicated reactor facilities,
4. The use of cheap, moderately reactive, and easy-to- handle reagents (iron and H2O2) makes the Fenton process cost effective and practically viable,
However, two main drawbacks were identified. The first is related to the wastage of oxidants due to the radical scavenging effect of hydrogen peroxide as in reaction (6) and its selfdecomposition as in reaction (12). The second refers to the continuous loss of iron ions and the formation of solid sludge. Several economic and environmental drawbacks have been reported to occur with Fenton sludge [3,37]. Thus, technologies allowing an efficient use of H2O2 have to be studied. Furthermore, an attempt has to be made for the recovery of iron ions and their subsequent recycle and reuse. Although Fenton's reagent was discovered about 100 years ago, its application as an oxidizing process for destroying toxic organics was not applied until the late 1960's [3,38].
Homogeneous vs. Heterogeneous Systems
Iron species exist in the same phase with reactants in homogeneous Fenton reaction. Therefore, there is no mass transfer limitation. A large number of studies have been conducted successfully using iron salts in Fenton processes for treatment of various wastewaters. Despite significant mineralization efficiency of homogeneous Fenton processes under optimum condition, a number of limitations are associated with these processes. The main drawback is the formation of large quantity of ferric-hydroxide sludge at pH values higher than 4.0 [39], that poses in adverse effects on the environment and waste disposal issues. In addition, regeneration of catalyst is not only impracticable but also large amount of catalytic metal is misplaced in the sludge. These limitations can be overcome to some extent by application of heterogeneous catalysts. This category of catalysts has gained growing concern in Fenton process as its effectiveness is maintained for wider range of pH. Iron is stabilized within the catalyst structure in heterogeneous catalysis and can effectively activate degradation of recalcitrant compounds without generation of ferric hydroxide precipitation. Nevertheless, heterogeneous catalysis is of slower oxidation rate compared to homogeneous reaction [40] due to the presence of a small fraction of iron on the catalyst surface. On this basis, recent investigations have focused on the development of new hetero-catalysts with larger surface area and higher activities in degradation processes [3,39]. Three possible mechanisms have been proposed for hetero-catalysts action in Fenton processes:
1. Iron leaching to the reaction solution and activating hydrogen peroxide through homogeneous pathway and/or
2. Decomposition of H2O2 to hydroxyl radicals by binding of H2O2 with iron species on the surface of catalyst and its decomposition to hydroxyl radicals or
Numerous heterogeneous catalysts have been used in Fenton reactions. Amongst them are iron minerals that are relatively less priced and can be separated magnetically from the reaction medium [43]. Details related to the application of different iron oxides in Fenton reactions and their degradation efficacy have been reviewed previously by the authors of this paper [34,39]. In addition, application of ferrites, clays, zeolite, alumina, fly ash based catalysts and other types of heterogeneous catalyst have been reviewed in detailed by other researchers [43-46]. Finally it should be pointed out that heterogeneous Fenton-like treatment of highly polluted wastewaters with low transparency is not practically efficient because of inner filtration effects related to large number of absorbing molecules and inhibition of photons absorption by iron cations [39,47].
Influence of Operational Condition
Amongst various factors that influence the effectiveness of degradation process in the Fenton oxidation system, the concentrations of the contaminant and Fenton reagents, pH and temperature of the reaction medium are found to be the most significant factors. In this context, the optimization of the reaction is very important to achieve better treatment results.
Structure of contaminants
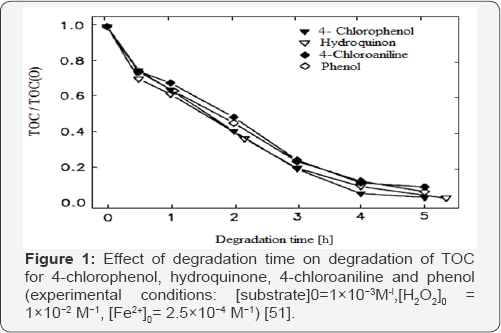
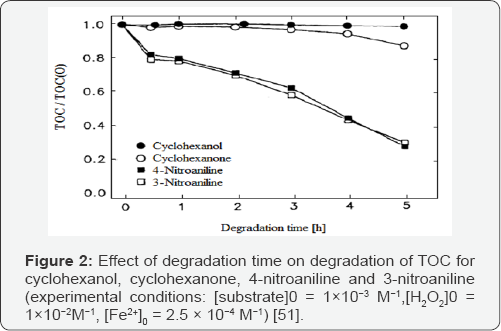
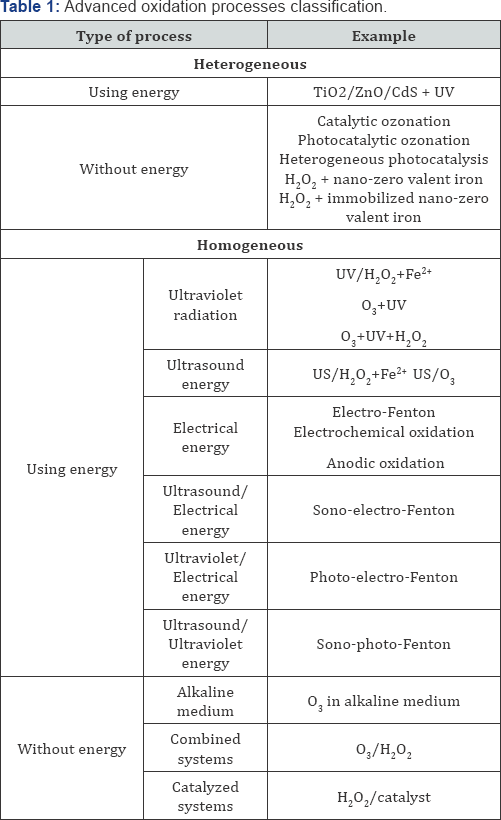
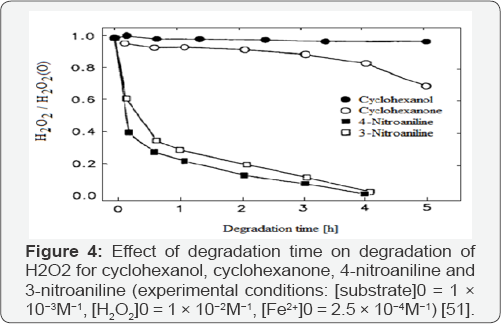
Kinetic degradation of aromatic pollutants with the Fenton system was reported earlier [48-50]; but less attention was given to the mineralization of these substances. The degradation of alicyclic compounds was given little attention since most of the water pollutants with a low biodegradability have an aromatic structure. The influence of the structure of several organic pollutants on the way they are mineralized by hydroxyl radicals has been studied [11,51]. As shown in (Figures 1 & 2), all of the aromatic substances studied by authors were strongly degraded after several hours, while the organic carbon of cyclohexanol and cyclohexanone was hardly attacked. In alicyclic compounds the attack of the electrophilic hydroxyl radicals cannot occur at conjugated C=C double bonds in contrast to aromatic compounds where ring opening and further degradation take place. As shown in (Figures 3 & 4), H2O2 decrease during reaction was in good correlation with the TOC (total organic carbon) degradation. For all aromatic substances studied degradation curves became linear after the first 30 min, until H2O2 was completely exhausted. During degradation of cyclohexanol and cyclohexanone only a slight decrease of the oxidant could be observed [11]. The continued destruction of nitroaniline after exhaustion of H2O2, as shown in (Figure 2) was attributed to photo-Fenton reactions [51]
Probe Molecules
The contaminant concentration is one of the important factors in Fenton process dealing with photons. Literature survey has clearly revealed that the increase in concentration of probe molecule has negative effects on its removal efficiency due to the inner filtration effect related to high concentrations of absorbing molecules [52]. Consequently, it needs longer irradiation time and/or further magnification in Fenton reagents to supply adequate hydroxyl radicals into the reaction [39,53].
Operating pH
The main disadvantage often associated with homogeneous Fenton system is its pH dependency to achieve the best degradation efficiency [54]. This is a challenging issue especially in natural waters or highly buffered wastewaters. Fenton process is strongly dependent on the solution pH mainly due to iron and hydrogen peroxide speciation factors. The optimum pH for the Fenton reaction was found to be around 3, regardless of the target substrate [55-58]. The activity of Fenton reagent is reduced at higher pH due to the presence of relatively inactive iron oxohydroxides and formation of ferric hydroxide precipitate [59]. In this situation, less hydroxyl radicals are generated due to the presence of less free iron ions. The oxidation potential of hydroxyl radicals decreases with increasing pH. In addition, auto-decomposition (reaction (12)) of hydrogen peroxide is accelerated at high pH [3,60]. In general higher-than-optimum pH values disturb Fenton efficiency through:
1. Prevention of H2O2 decomposition to generate hydroxyl radicals due to the deficiency of H+ ions [18],
2. Accelerated decomposition of H2O2 to water and oxygen at pH values above 5,
3. Decline in oxidation potential of hydroxyl radicals (E0 = 2.8-1.95 V at pH 0-14) [61],
4. Possible generation of more selective ferric species other than hydroxyl radicals at pH above 5 [62] and
At pH below 3, decrease in degradation efficiency was observed [66]. At very low pH values, iron complex species [Fe(H2O)6]2+ exists, which reacts more slowly with hydrogen peroxide than other species [67]. In addition, the peroxide gets solvated in the presence of high concentration of H+ ions to form stable oxonium ion [H3O2]+. Oxonium ions make hydrogen peroxide more stable and reduce its reactivity with ferrous ions [3,66,68]. Therefore, the efficiency of the Fenton process to degrade organic compounds is reduced both at high and low pH. Thus an adequate control of pH would increase process efficiency. It should be noted that the type of buffer solution used also has effect on the degradation process [69]. The acetic acid/ acetate buffer gives maximum oxidation efficiency whereas least is observed with phosphate and sulfate buffers [69]. This can be attributed to the formation of stable Fe3+ complexes that are formed under those conditions [3,70].
Ferrous ion concentration
The Fenton reaction begins by producing hydroxyl radicals from the reaction between ferrous ion and hydrogen peroxide (reaction (1)). When the Fenton reaction in the absence of organics is initiated under [Fe2+]0/[H2O2]0≥2, the consumption ratio of ferrous ion to hydrogen peroxide becomes about 2, and radical chain reactions are quickly terminated. This is because the hydroxyl radicals produced as a result of reaction (1) mainly react with the ferrous ion and not hydrogen peroxide. This explanation is supported by the fact that the reaction between hydroxyl radicals and the ferrous ion is ten times faster than that between hydroxyl radicals and hydrogen peroxide (k2 = 3.2 х 108M-1 s-1and k6 = 3.3 х 107M-1 s-1)[11,17].
Usually the rate of degradation increases with an increase in the concentration of ferrous ion [71]. However, the extent of increase is sometimes observed to be marginal above a certain concentration of ferrous ion [55,72,73]. Also, an enormous increase in the ferrous ions will lead to an increase in the unutilized quantity of iron salts, which will contribute to an increase in the total dissolved solids content of the effluent stream and this is not permitted. Thus, laboratory scale studies are required to establish the optimum loading of ferrous ions to mineralize the organics [3].
Hydrogen peroxide concentration
Concentration of hydrogen peroxide plays a crucial role in deciding the overall efficiency of the degradation process [3]. It has been observed that the degradation percent of the pollutant increases with an increase in the dosage of hydrogen peroxide [56,71-73]. However, care should be taken while selecting the operating oxidant dosage. The unused portion of hydrogen peroxide during the Fenton process contributes to COD [71] and hence excess amount is not recommended. Also, the presence of hydrogen peroxide is harmful to many of the organisms [74] and will affect the overall degradation efficiency significantly, where Fenton oxidation is used as a pretreatment to biological oxidation [3].
With hydrogen peroxide concentrations at an average [Fe2+]0/[H2O2]0 ratio =1,regardless of the presence of organics, hydrogen peroxide rapidly converts all ferrous to ferric ions via reaction (1). In the absence of RH, hydrogen peroxide decomposes slowly through ferric ion induced radical chain reactions (reaction (3)) just after the rapid consumption of hydrogen peroxide. The reduction of the ferric ion (reactions (3)) is significantly lower than reaction (1) and is the rate- determining step. To have a continued decrease of hydrogen peroxide, ferrous ion must be formed by the reduction of ferric ion. Then, the Fenton reaction can be characterized by two specific systems, i.e. the ferrous system and the ferric system, which depend on the oxidation stage of the iron initially added or the major oxidation state of the iron present. The ferrous system refers to the case where the primary reaction, which produces hydroxyl radical, is the reaction between the ferrous ion and hydrogen peroxide (reaction (1))[11,12].
Initial concentration of pollutant
In general, lower initial concentration of the pollutants is favored [68,69], but the negative effects of treating large quantities of effluent need to be analyzed before the dilution ratio is fixed. For real industrial wastewaters, dilution is essential before any degradation is effected by Fenton oxidation [3].
Temperature
A large number of studies related to Fenton-based methods have been carried out at room temperature [39,75-77]. This is because thermal decomposition of H2O2 occurs at temperatures above 50°C [78-84]. In addition, due to the fact that H2O2 decomposition is accelerated at basic pH values, the increment in temperature brings about a shift in optimum pH towards acidic values [39,75,80]. In fact, a study [71] reported an optimum temperature of 30°C, whereas another study [55] reported that the degradation efficiency is unaffected even when the temperature is increased from 10 to 40°C. If the reaction temperature is expected to rise beyond 40°C due to exothermic nature, cooling is recommended. The efficient utilization of hydrogen peroxide decreases due to accelerated decomposition of hydrogen peroxide into water and oxygen [3,81]. However, based on the Arrhenius theory of rate constants in relation to temperature, it is expected that increase in temperature leads to higher generation of hydroxyl radicals from the increase in concentration of produced Fe(OH)2+ [82]. In another study, complete mineralization of diclofenac, a phenyl containing drug compound was attained when the experiments were carried out at 50°C [83]. However, the authors did not report any data regarding degradation efficiency at ambient temperature [39].
Chemical coagulation
Chemical coagulation step is recommended after Fenton oxidation to keep the concentration of the soluble iron with the specified limits [71]. A study [72] has demonstrated the efficacy of chemical coagulation in controlling the concentration of total dissolved solids below the specified limits [3].
Composition of Reaction Medium
Besides main operational conditions, there are several other factors that affect the efficiency of Fenton and Fenton-based oxidations. Amongst them is the composition of the treated water. Inorganic ions such as carbonates, bicarbonates, chlorides, fluoride, bromide, phosphate and sulfate may be present in water or generated via degradation process. Some of these ions may alter oxidation rate of Fenton reactions [39]. The extent of the change in reaction kinetic depends on the type of ion and its concentration in the solution through one or combination of the following effects:
• formation of Fe(III) complexes and lessening of the abundance and activity of iron species,
• generation of by-products that are in some cases more toxic and recalcitrant than parent compounds,
• hydroxyl radical scavenging and generation of less reactive radicals than hydroxyl radicals,
• reaction of generated radicals with hydrogen peroxide that decreases its availability in solution,
• competition with organic compounds for active sites on hetero-catalysts, and
Chelating Agents
Despite the fact that Fenton processes provide acceptable scores for contaminant degradation in acidic solutions, a large number of recent works have employed several inorganic or organic ligands such as EDTA, EDDS, oxalate, NTA, carboxymethyl b-cyclodextrin (CMCD), tartrate, citrate and succinate, to improve its efficiency and to increase the oxidation rate of probe molecules [39,91-94]. The positive effects of these ions can be attributed to the following aspects:
• having higher quantum yield of hydroxyl radical formation compared to other Fe (III) complexes,
• promoting the reduction of ferric ion to ferrous ion and accordingly, regeneration of higher amounts of hydroxyl radical [39,95,96],
• promoting H2O2 activation and hydroxyl radical generation,
• enhancing the solubilization of lipophilic organic pollutants, and
Energy-Consuming Fenton-Based Reactions
Photo-Fenton processes
A combination of hydrogen peroxide and UV radiation with Fe2+ or Fe3+ oxalate ion (photo-Fenton (PF) process) produces more hydroxyl radicals compared to conventional Fenton method or photolysis and in turn increases the rate of degradation of organic pollutants [97-104]. Fenton reaction accumulates Fe3+ ions in the system and the reaction does not proceed once all Fe2+ ions are consumed. The photochemical regeneration of ferrous ions (Fe2+) by photo-reduction (reaction (13)) of ferric ions (Fe3+) occurs in photo- Fenton reaction [105]. The newly generated ferrous ions react with H2O2 and generate hydroxyl radical and ferric ion, and the cycle continues [3]:
FeOH2++hv → Fe2++ OH. -----(13)
The studies reported in the literature showed that the combination of Fenton reaction with conventional radiation zone of the visible and near ultraviolet gives a better degradation of organic pollutants. Pollutants such as 4-chlorophenol [101], nitrobenzene and anisole [102], herbicides [98] and ethylene glycol [103] were degraded effectively by photo-Fenton process [3].
A study [104] compared the degradation of two commercial anionic surfactants such as sodium dodecyl sulfate and dodecylbenzenesulfonate, using Fenton reagents (Fe2+ or Fe3+ with H2O2 in the presence or absence of solar radiation), photocatalysis (TiO2 with solar irradiation) and photo-degradation using solar sensitizer (pyrylium salt). They demonstrated that the addition of the solar sensitizer did not efficiently degrade the surfactants and their further studies concluded that the photo- Fenton processes using solar radiation (0.1 mM of Fe2+ or Fe3+, and 1 mM H2O2) had a higher rate of surfactant degradation than that of solar-TiO2 treatment [3].
Sono-Photo-Fenton Process
The combined treatment using ultrasound and ultraviolet along with Fenton reagent is known as sono-photo-Fenton (SPF) process, which enhanced the production of hydroxyl radicals in an aqueous system significantly. Sonolysis of water produces hydroxyl radicals and hydrogen atoms. However, significant loss of H .and hydroxyl radical species occurs due to the recombination. On the other hand, the applications of UV light, converted the hydrogen peroxide produced by recombination of hydroxyl radicals, and in turn increased the amount hydroxyl radical [105]. The intermediate complex formed due to the reaction of Fe3+ with H2O2 during the Fenton reaction could be reduced to Fe2+ by sonolysis [106] and photolysis [3,107]. The degradation of recalcitrant pharmaceutical micro-pollutant ibuprofen (IBP) by means of sono-photo-Fenton, sono- photo-catalysis and TiO2/Fe2+/sonolysis processes has been investigated [108]. The presence of ultrasound irradiation in photo-Fenton process improved the iron catalytic activity and ibuprofen degradation and mineralization to 95% and 60%, respectively. On the other hand, total removal of ibuprofen and elimination of more than 50% of dissolved organic carbon were observed by photo catalysis with TiO2 in the presence of ultrasound irradiation [3]. The results showed that, the hybrid system is a promising method for complete elimination/ mineralization of the recalcitrant micro-contaminant ibuprofen.
Sono-electro-Fenton process
Many researchers have reported the coupling strategy between sonochemistry and different AOPs such as the Fenton process giving rise to the concept of advanced sonochemical hybrid techniques that possess significantly greater efficacy for water remediation [109-111]. Hydroxyl radicals produced by water decomposition are used for the degradation of organics [3,111]. The effect of low frequency ultrasonic irradiation on the sono-electro-Fenton oxidation of cationic red XGRL has been evaluated [112]. Ultrasonic irradiation significantly increased the hydrogen peroxide production rate and reduced the time needed to reach the maximum hydrogen peroxide concentration. In addition, ultrasonic irradiation has a considerable effect on the degradation of cationic red X-GRL. The results showed that the degradation rate followed pseudo-first order kinetics and also decolorization rate increased with ultrasonic power. Furthermore, total organic carbon removal efficiency and mineralization were greatly promoted in sono-electro-Fenton process compared to electro-Fenton process [3]. These results proved that sono-electro- Fenton process is a promising technology in terms of colored wastewater treatment.
Photo-electro-Fenton process
The catalytic effect of Fe2+ in the electro-Fenton process can be enhanced by irradiating the contents with UV light. Thus, the combination of electrochemical and photochemical process with Fenton process is called photo-electro-Fenton process generates greater quantity of free radicals due to the combination effect [113,114]. The direct photolysis of an acid solution containing peroxide generates hydroxyl radicals through the breakdown of the peroxide molecule according to reaction (14). This reaction increased the oxidative capability of the process due to the additional production of hydroxyl radicals. Thus, the degradation of target organic substrate can be enhanced when the solution is irradiated with UV light in addition to the application of electro- Fenton process. Photochemical regeneration of Fe2+ by the photo reduction of Fe3+ ions and photo-activation of complexes renders the photo-electro-Fenton systems more efficient [3,115,116]. At acidic pH, oxalic acid derivatives behave as the photo-active complexes in the presence of ferric ions which undergo photodecarboxylation reaction [19] as shown in reaction (15).
H2O2+hv →2OH.---- (14)
R(CO2)Fe(III)+ hv →RCO2.+Fe(II)+ R.+CO2 ---- (15)
The studies pertaining to the application of photo-electro- Fenton process are very limited and most of the studies are related to the treatment of herbicide [113,115,117], 4-chloro-2- methylphenol [118] and dyes [119]. In another study [120] solar photo-energy is recently used as photon source and reduced the operating costs of the process substantially [3].
Application of Fenton and Fenton Based Systems in Industries
There have been extensive studies on application of Fenton and Fenton-based systems for treatment of various industrial wastewaters and synthetic recalcitrant solutions at laboratory or pilot plant scales. Amongst them are dyes which are the major organic pollutants. Photo-Fenton treatment of synthetic solutions of dyes such as acid blue, acid orange 7 and acid red 151 [121], remazol red RR [40], orange II [122], procion red H-E7B and cibacron red FN-R [123] and 218 also real dye wastewaters [124-127] are examples that have recently been reported. In addition, Fenton-based systems application exclusively or in combination with other processes for treatment of industrial wastewaters such as winery [128], pulp mill [129], cork boiling [130], plastic containers washing [131], pharmaceutical [132] and alkydic resins [133] have been well documented. Despite a large number of studies on dye/textile wastewaters treatment by homogeneous and/or heterogeneous Fenton-based systems, it has been reviewed previously by a number of researchers [39,134]. Accordingly, this part of the present review focuses on the application of these oxidation processes for treatment of pharmaceuticals, agrochemicals and petroleum refinery effluents.
Pharmaceuticals
Pharmaceutical plants generate wastewaters containing toxic solvents and intermediates that are usually lipophilic and non-biodegradable in regard to accepting media. Besides pharmaceutical manufacturing discharges, other sources are effluents containing personal care products, hospital wastewaters and veterinary effluents. Inappropriately treated pharmaceutical effluents results in several problems such as occurrence of antibiotic-resistant bacteria, interference in human endocrine system and feminization of higher organisms [135-138]. However, the potential effects of a large number of these active chemicals in co-existence with other chemicals on human being and other living organisms are not entirely understood [39,139]. Literature has shown that the effectiveness of conventional treatment methods for recalcitrant pharmaceutical wastewaters is limited. In contrast, AOPs have shown great ability for oxidizing and mineralizing many non- biodegradable pharmaceuticals. Various AOPs such as ozonation [140], sonolysis [141], UV/H2O2 system [142], wet air oxidation [143], Fenton oxidation [144] and photo-Fenton process have been applied for pharmaceuticals degradation in water and wastewater [39,145].
Although pharmaceutical wastewater treatment by homogeneous Fenton-based processes has been reported as one of the most appropriate methods amongst AOPs, its degradation efficiency depends on COD: H2O2:Fe2+ ratio and the pH range of 2.5-4 [142]. Trovo et al. [146] studied paracetamol, a pain reliever drug, degradation by solar Fenton-based processes using FeSO4 and potassium ferrioxalate (Fe-Ox). The degradation was enhanced with FeSO4 as compared to Fe-Ox. This can be related to the effects of FeSO4 in forming large amount of hydroxylated intermediates and accordingly, increasing in the generation of Fe2+ through Fe3+ reduction. On the other hand, complete degradation of amoxicillin (AMX) was obtained using Fe-Ox with only 5 min of irradiation, in comparison with FeSO4 that took 15 min [147]. Kajitvichyanukul and Suntronvi part [148] employed photo-Fenton process as a pre-treatment step to enhance the oxidation degree of hospital effluents at laboratory scale. Complete COD removal was obtained by sequential activated sludge treatment which is a less costly post-treatment method [39].
Agrochemicals
The increase in food and fiber production has always been connected to the use of pesticides [149]. Pesticides including herbicides, insecticides and fungicides are used based on their specific biological activity on target species [150]. Besides cultivated areas, one of the most important sources of pesticide contamination is discharges from pesticide production plants [151]. In general, pesticides affect the health of living organisms directly and are toxic and carcinogenic in nature even at microconcentrations [152]. Generally, pesticides are removed from industrial wastewaters by physical-chemical methods due to the shock doses associated with pesticides and their toxicity to microbial cultures [153]. Literature reviews confirm Fenton- based processes as effective methods for degradation of recalcitrant agrochemicals. In addition, Fenton-based processes have been reported as one of the most appropriate pretreatment/treatment systems compared to other AOPs [39]. For example, Teixeira et al. [154] carried out aphoto-Fenton process along with (H2O2/UV), (TiO2/UV) and conventional Fenton for decontamination of wastewaters containing active compounds of a fungicide (tebuconazole) and an insecticide (methamidophos) at laboratory scale. The results indicated that the photo-Fenton process performed better compared to other AOPs at all tested Fe2+ and H2O2concentrations.
In addition, Fenton-based processes were able to reduce dissolved organic carbon (DOC) and COD to 32% and 27% respectively after 60 min. Methomyl is known as a highly toxic carbamate insecticide [155] that is highly soluble in water. The study on the removal efficiency of this toxic chemical using Fenton and Fenton-based processes was carried out by Tamimi et al. [65]. Complete degradation of methomyl was attained after 30 min of irradiation whereas its removal degree with Fenton oxidation was 86.1% after 60 min. Lower reaction time (3.96 min vs. 13.86 min) and higher oxidation rate (0.1750 min-1 vs. 0.0500 min-1) were observed for Fenton-based processes compared to the Fenton process. In another study, Maldonado et al. [156] compared the efficiency of photo-Fenton with TiO2 photo catalysis and Fenton process for degradation of a number of pesticides (alachlor, atrazine, chlorfenvinphos, diuron, isoproturon and pentachlorophenol) and a mixture of them. The authors reported photo-Fenton process as the most suitable system for mineralization of these compounds and their mixture with the advantage of shorter reaction time (38-79%) compared to TiO2 photo catalysis. In addition, total TOC removal was obtained in Fenton-based systems after less than 15 min of irradiation, while no mineralization of atrazine and isoproturon took place using Fenton process. The photo-reactivity of the pesticides was reported in the following order: diuron > alachlor > isoproturon > chlorfenvinphos > atrazine with similar behavior in both mixture and individual treatments [39].
Petroleum Refinery plant
Petroleum refinery plant transforms crude oil into multitude refined products. Besides air pollution, the generated effluent is of concern [157]. Large amounts of water, that is about 0.41.6 times the quantity of the processed crude oil, is used for refining processes of cooling system, distillation, hydro-treating, and desalting [158]. This wastewater may expose various contaminants into the aquatic environment. Petroleum refinery effluent (PRE) has high concentrations of aliphatic and aromatic compounds especially polycyclic hydrocarbons along with oil and grease [159] in which aromatic fraction is more toxic and recalcitrant than aliphatic portion [160-163]. The aromatics are therefore the principal issue in degradation due to their toxicity and potential hazards. Generally, PREs are characterized by high COD, low biodegradability (BOD5/COD <0.4) and large fraction of high molecular-weight organic compounds [158,163,164]. Several treatment technologies such as adsorption [165], coagulation and flocculation [166], bioremediation [167], electrochemical processes [168], membrane technology [169] and different advanced oxidation processes of wet air oxidation [170], photolysis [160], Fenton [104] and photo-Fenton [158,171] have been used for petroleum refinery wastewaters.
However, biological treatment has exhibited poor performance for complete removal of refractory chemicals from PREs [172]. Coelho et al. [158] carried out Fenton-based processes for DOC removal of petroleum refinery sour water. They conducted batch oxidation experiments in a two-step (Fenton and photo-Fenton) and one-step (photo-Fenton) process. There were 87% and 70% DOC removal, demonstrating improvement in degradation with a decrease in energy consumption in treatment process by applying combined processes [39]. Photo- Fenton oxidation system was also tested on treatment of water contaminated by diesel oil in a study by Galvao et al. [171]. In this study, although the amount of ferrous ion was very low (0.1 mmol L-1), the TOC removal was up to 99%. However, only 28% and 26% mineralization of diesel oil were obtained using UV photolysis and thermal Fenton oxidation. Nevertheless, longer irradiation time in UV/H2O2 process could increase the mineralization up to 71%. Similarly, da Silva et al. [173] reported 89% of TOC removal in a wastewater containing xylene by applying 0.26 mmol L-1 Fe2+ when the allowable limit of Fe2+ was 0. 27 mmol L-1[39].
Practically, it is not conceivable to judge the effectiveness of all Fenton-based processes for PREs based on the present available studies. More studies on simulated and real PREs with complex matrices, instead of one organic compound, is required to their effectiveness on comparison. In addition, due to complex nature of PREs, combination of Fenton or Fenton-based processes with a series of pretreatment and/or post-treatment processes will be helpful to achieve cost effective and acceptable results [39].
Transition Metal Substituted Iron Oxide Catalysts
Since both homogeneous and heterogeneous iron-based Fenton AOPs show severe practical disadvantages, research efforts are being focused on finding new practically acceptable and economically-viable Fenton catalysts to generate hydroxyl radical from H2O2. In this regard, to achieve an efficient electron transfer to H2O2, the ideal Fenton catalyst should exhibit multiple oxidation states because the catalytically-active species with a specific oxidation state can be easily regenerated from an inactive form through a simple redox cycle. To achieve this objective, both active and inactive redox states should be stable over a wide pH range to prevent the precipitation of the catalytic species. Elements with multiple oxidation states efficiently decompose H2O2 even at neutral pH in both homogeneous and heterogeneous reaction conditions. While the exact activation mechanism is strictly dependent on the nature of the catalyst, it is effectively controlled by solution pH and/or metal-ligand complexation. Importantly, redox transformation of these non- ferrous metal species is easily achieved using the pH-dependent dual role of H2O2 as both oxidant and reductant [1].
Aluminum
In homogeneous aqueous solution, the only accessible oxidation state for aluminum (Al) is Al3+. Thus, unlike the case of iron with both Fe2+ and Fe3+ states, the electron transfer reaction between Al3+ and H2O2 is not possible. On the other hand, the charge transfer using zero-valent aluminum (Al0 or ZVAl) as the electron source is thermodynamically more efficient: ZVAl [E0(Al3+/Al0) = -1.66 V] provides a much stronger thermodynamic driving force for the electron transfer to H2O2 [E0(H2O2/HO.) = 0.8 V at pH 7] compared to Fe0 [E0(Fe2+/Fe0) = -0.4-4 V] or Fe2+ [E0(Fe3+/Fe2+) = +0.776 V] . This enhanced electron transfer capacity of ZVAl was first demonstrated in 1991 to reduce nitrate to ammonia in aqueous solution [174]. Ten years later, Lien and co-workers [175,176] reported the oxidation of organic substrates using electron transfer from surface-functionalized aluminum metal. Using bifunctional aluminum prepared by sulfated Zale with sulfuric acid, electron transfer to molecular oxygen (O2) generated reactive oxygen species (ROS) for the oxidation of methyl tert-butyl ether (MTBE) and tert-amyl methyl ether (TAME) [1]. The sulfate species on the ZVAl surface enhanced the oxidation efficiency by first stabilizing the ROS on the active sites and then adsorbing the organic substrate to initiate oxidation.
The use of ZVAl to decompose H2O2 into hydroxyl radicals for pollutant oxidation has major advantages such as high natural abundance (most abundant metal in earth's crust) and low weight (three times lighter than Fe). However, the surface Al2O3 layer cannot be easily removed in neutral or near-neutral pH conditions, which restricts the practical applications of ZVAl-based AOP systems to strictly acidic environments (below pH 4), similar to Fe based Fenton processes. Nevertheless, the ZVAl aerobic system still exhibits significantly higher oxidative capacity compared to Fe-based one, mainly owing to the high reduction potential and the enhanced aqueous solubility of Al3+ species [177]. Thus, within the same practical limitations (acidic effluents only), the use of ZVAl offers an efficient alternative for ex situ oxidation processes [1].
Cerium
Among all rare-earth or lanthanide group elements, cerium is the only metal capable of activating H2O2 by Fenton-like mechanism. Due to its 4f2 6s2 valence configuration, cerium is the only rare earth element to exhibit both +3 and +4 oxidation states in solution. While the cerous (Ce3+) form is a strong reducing agent and easily oxidized by O2 in alkaline condition, the ceric (Ce4+) species is a strong oxidant under acidic condition. Thus, cerium can easily cycle between the Ce3+ and Ce4+ oxidation states under suitable redox conditions [E0(Ce4+/Ce3+) = +1.72 V]. To exploit this simple redox transformation for catalytic applications, cerium oxide (CeO2 or ceria) is the most popular choice among all cerium compounds. Owing to the presence of oxygen vacancies on the oxide surface, the availability of surface Ce3+ at such defect sites is enhanced and induces high catalytic activity [178]. In addition, the easy formation and elimination of oxygen defects [179] during catalytic applications allow the repeated redox cycles of Ce4+/Ce3+ on the particle surface. Thus, CeO2 is widely used in catalytic wet air oxidation, water gas-shift reaction, and three-way automobile exhaust converters [1,180].
A series of in-depth investigations of the CeO2/H2O2 Fentonlike system by Chen and coworkers [181-184] revealed that the production of hydroxyl radicals (and overall oxidation mechanism) critically depends on the oxide surface properties. Without any surface modification, the reaction between Ce3+ and H2O2 leads to the formation of stable brown peroxide-like species (=Ce3+-OOH-), which remain stable even at neutral pH and do not directly decompose to generate free hydroxyl radicals [182, 183]. Thus, bare cerium oxide is incapable of Fenton-like oxidation based on hydroxyl radicals [1]. However, these peroxide species easily decompose into hydroxyl radicals when cerium oxide is pre-treated using sulfuric acid (sulfated). The CeO2/ H2O2 heterogeneous redox system can be easily manipulated by simple surface modification to efficiently generate hydroxyl radicals under mild acidic condition. Due to the critical role of surface Ce3+ species on the catalytic efficiency, the use of nanosized ceria particles will further amplify the effective surface concentration of Ce3+ and increase the overall hydroxyl radical's yield. However, considering the acute cytotoxicity of cerium (both ionic and oxide forms) to aquatic life, plant species, and human beings [185], the catalytic stability and post-treatment disposal of cerium oxide catalysts need to be thoroughly examined before practical applications [1].
Chromium
Chromium (Cr) can theoretically exist in multiple oxidation states (from -2 to +6) but only the trivalent [Cr(III)]and hexavalent [Cr(VI)] species are commonly detected in water. Under the Eh- pH range of natural water, the cationic Cr(III) is the prevalent species at low Eh and pH values, whereas Cr(VI) exists as anionic CrO42- or Cr2O72- in oxidizing conditions. While Cr(III) is an essential micronutrient for animals and plants and is critically involved in sugar metabolism [186], Cr(VI) is a Group 'A' human carcinogen and causes mutagenic liver damage, pulmonary congestion, and allergic dermatitis [187]. This contrasting toxicity profile is critically dependent on the aqueous solubility characteristics of both chromium species. The trivalent Cr(III) is easily precipitated as insoluble chromium hydroxide [Cr(OH)3] in neutral and alkaline conditions (pH > 5), but the hexavalent Cr(VI) is completely water soluble in the entire pH range [1]. Despite their different aqueous solubility behavior and inherent toxicity, both trivalent and hexavalent chromium species react strongly with H2O2 and generate hydroxyl radicals via a series of Fenton-like processes.
The reaction between Cr(VI) (present as oxyanion CrVIO42-) and H2O2 initiates the replacement of oxo ligands by peroxo groups and one-electron reduction of the metal center to form a [CrV(O2)4]3- complex, which subsequently decomposes to form hydroxyl radicals and regenerate Cr(VI) [187-189]. Although the dissociation of this Cr(V)-complex into hydroxyl radicals is strongly favored at acidic pH, the oxidation of various organic pollutants was achieved even in neutral and near-alkaline conditions [188]. This indicated the formation of a reactive Cr(V) intermediate in a wide pH range (3.0-9.0), which is an advantage of using Cr(VI) for Fenton-like activation of H2O2. The high aqueous solubility of Cr(VI) is also an additional merit for using Cr(VI)/H2O2 as a homogeneous AOP. However, despite these practically-favorable reaction conditions, the extreme toxicity of Cr(VI) prohibits any deliberate addition into wastewater treatment. Nevertheless, the Cr(VI)/H2O2 redox system is ideal for the treatment of wastewaters already co-contaminated with Cr(VI) [1] and organic compounds such as effluents from leather tanning, electroplating and petroleum refining industries.
Cobalt
Divalent cobalt ion (Co2+) has been widely investigated as a Fenton-like catalyst for the oxidation of organic pollutants. Using the Co2+/Co3+ redox couple [E0(Co3+/Co2+) = +1.92 V], a majority of studies have largely focused on the activation of persulfate (S2O82-) or peroxymonosulfate (HSO5-) to generate sulfate radicals (SO4.-) as the main oxidant species [1]. Despite the fact that Co2+ demonstrates the best efficiency for SO4.- generation compared to all other transitional metals [190,191], the generation of hydroxyl radicals by Co2+-mediated activation of H2O2 has been documented only in a few studies.
Ling et al. [192] first investigated the homogeneous Co2+/ H2O2 redox system for the complete oxidation of basic blue dye and reported a strong correlation between [H2O2] and degradation efficiency. Similar studies using photo-Fenton [193] and electro-Fenton [194] processes based on soluble Co2+ salts and H2O2 also demonstrated efficient oxidation capacity. However, the existence of a Fenton-like oxidation pathway via formation of hydroxyl radicals was not discussed or confirmed in all three reports. On the other hand, the oxidation of organic pollutants by heterogeneous Co2+ catalysts like Co2+/Al2O3 [195], Co2+/MCM-41 [196] and Co2+/carbon aerogel [197] was also achieved in the presence of H2O2. The proposed reaction mechanism suggested the formation of a colored peroxo-cobalt complex on the catalyst surface [1], and further reaction with the organic substrate to generate organic radicals (reactions 16-18) [195]:
SCo2+(H2O)4+ H2O2 ↔SCo2+ (H2O)4 OOH+ H+ ---- (16)
SCo2+ (H2O)4 (OOH)+ R→ SCo2+(H2 O)4+ROOH.(or R). ---- (17)
SCo2+ (H2O)4+ H2O2 → SCo2+ (H2O)4+ 1/2 O2+ H2O ----- (18)
Where >S refers to the support matrix and R denotes organic pollutant. However, the decomposition of peroxo-cobalt complex to generate hydroxyl radicals and its possible involvement in the overall oxidation pathway (HO. + R → R.) was not explored [1].
Copper
In terms of its reactivity towards H2O2, copper shows strikingly similar redox properties like iron. Both the monovalent (Cu+) and divalent (Cu2+) oxidation states react easily with H2O2 (reactions (19) and (20)), analogous to the Fe2+/H2O2 and Fe3+/ H2O2 reaction systems, respectively [1].
Cu2++ H2O2 → Cu++ HO2.+OH ----- (19)
Cu2++ H2O2 → Cu2++ OH.+OH ----- (20)
The hydrolyzed complex with the most stable oxidation state, i.e., Cu(OH)2 for Cu2+ and FeOH(H2O)2+ for Fe3+, are both Fenton-active catalysts [198]. However, there is a strong difference in the aqueous solubility characteristics of Cu2+ and Fe3+. While the iron aquo complex [Fe(H2O)6]3+ is insoluble at pH > 5, the corresponding copper complex [Cu(H2O)6]2+ is predominant in neutral pH conditions [199]. This means that [1] the Cu2+/H2O2 Fenton-like system should work over a broader pH range, compared to the Fe3+/H2O2 redox system working only in the acidic condition. Accordingly, all Cu2+- based Fenton catalysts efficiently generate hydroxyl radicals for the oxidation of various organic pollutants in near-neutral or neutral aqueous solutions. Additionally, Cu2+ complexes with organic degradation intermediates (organic acids) are easily decomposed by hydroxyl radicals, whereas, the corresponding Fe3+ complexes are highly stable [200,201]. As a result, unlike Fe3+- based systems, Cu2+ complexation does not block (or deactivate) the Fenton reaction nor prevents complete mineralization of organic pollutants [1,199]. Thus, considering the similarities with Fe3+/ H2O2 Fenton-like system combined together with high catalytic stability in neutral conditions, the cupric ion (Cu2+) satisfies all basic redox criteria required to activate H2O2 in large-scale practical applications.
Manganese
Compared to cobalt and copper, manganese (Mn) can exist in more variety of oxidation states ranging from 0 to +7. Although manganate (Mn6+) and permanganate (Mn7+) compounds are highly stable, only the oxidation states of +2 to +4 have environmental and catalytic significance. Both Mn2+ and Mn3+ compounds are water-soluble, whereas all naturally occurring manganese species (minerals and ores) contain predominantly Mn3+ and Mn4+ in oxide forms (Mn3O4, Mn2O3 and MnO2). However, manganese exists only as Mn2+ or Mn4+ in the aquatic environment and the "bioavailable" divalent form is stable only at low pH (pH < 4) and in reducing conditions [202]. In aerobic neutral conditions, the complete oxidation of Mn2+ to Mn4+ involves the intermediate formation of colloidal Mn3+- oxyhydroxides and subsequent conversion into MnO2 [1]. The tetravalent Mn species can be easily reduced to Mn2+ through chemical redox processes (reaction (21)), which makes MnO2 a powerful oxidant for direct transformation of aqueous toxins [203].
MnO2+S → Mn2++Oxidized-S -----(21)
Where S represents the organic/inorganic substrate. Therefore, the facile inter-conversion between Mn2+ and Mn4+ via intermediate Mn3+ species should enable the Mn-catalyzed Fenton-like activation of H2O2 [1]. Despite the large variations in catalyst properties and radical characteristics, the oxide reactivity does not change significantly in the pH range 3.5 7.0 [204]. Mn dissolution does not occur above pH 5.5, which highlights the stability and easy recyclability for repeated oxidation cycles [1]. Therefore, manganese oxides offer many advantages for heterogeneous Fenton applications such as:
• optimal performance in near-neutral conditions,
• selective formation of ROS by careful choice of oxide composition, and
• High natural abundance of structurally-different oxide compounds [1].
Ruthenium
Among the transition metal catalysts, ruthenium (Ru) is the only member of the platinum group metal that exhibits Fentonlike activity in the presence of H2O2. Although the possible oxidation states of Ru range from 0 to +8, only the divalent (Ru2+), trivalent (Ru3+) and tetravalent (Ru4+) oxidation states are commonly found. Ruthenium complexes have been widely investigated for various organic transformation reactions like olefin hydroxylation, alcohol dehydrogenation, water oxidation and alkene epoxidation [1,205]. However, only limited studies on the oxidation of organic pollutants using Ru-mediated H2O2 decomposition have been published so far [1].
Using the Ru3+/Ru2+ redox couple [E0(Ru3+/Ru2+) = +1.29 V], the oxidation of bisphenol A was achieved by Hu et al. [206] using Ru2+-polypyridyl complex immobilized on cation exchange resins Dowex-50W and Chelex-100. The reaction between Ru2+ and H2O2 efficiently generated hydroxyl radicals in the pH range 4.0-8.0 with the oxidation efficiencies increasing at higher pH. The use of resin supports not only prohibited the leaching of the Ru complex but also facilitated the repeated oxidation cycles and easy catalyst recovery. On the other hand, Rokhina et al. [207] investigated the oxidation of phenol using porous RuI3 catalyst (anhydrous powder form) as an activator of H2O2 [1]. Analogous to the Fe3+/H2O2 Fenton-like system, the in situ formation of Ru2+upon the reduction of Ru3+ initiated the decomposition of H2O2 into hydroxyl radicals. The high stability of these Ru catalysts prevents metal leaching and allows multiple catalytic cycles. The latter is more important since Ru is an expensive and rare element. Therefore, the practical use of the Ru-based Fenton systems may be limited to the cases requiring exceptionally high catalytic performance and specialized reaction conditions [1].
Conclusion
It is difficult to treat wastewaters from pharmaceutical, agrochemical and petroleum refinery plants effectively by conventional methods due to their recalcitrant nature and resistance to bio-degradation. Advanced oxidation processes are found to be an environmental friendly process for the degradation of refractory compounds. Among advanced oxidation processes, Fenton and Fenton-based reaction treatment processes are known to be very effective in the removal of many hazardous organic pollutants from environment.
The popularity of Fenton and Fenton-based advanced oxidation processes for wastewater treatment has been credited mostly to the choice of iron-based catalysts. However, because of the restricted solubility of iron species, research efforts have focused on the establishment of iron-free Fenton systems for the activation of H2O2. Iron oxides as heterogeneous catalysts demonstrated considerable improvements in Fenton reactions for contaminant removal from polluted medium/sites due to their higher activity under a broad range of pH in contrast with soluble iron salts. The conclusions derived from the various literature sources can be stated as follows:
1. Catalytic materials with multiple oxidation states and redox stability efficiently decompose H2O2 to generate HO even in neutral/alkaline conditions in both homogeneous and heterogeneous reaction conditions.
2. The cytotoxic nature of soluble species like cerium, chromium and cobalt seriously limits the extent of large-scale applications, with only restricted usage permitted in controlled reaction conditions.
3. The development of ultra-stable heterogeneous metal complexes with enhanced reactivity for H2O2 and zero metal leaching remains the focal point to establish practically-viable and environmentally-sustainable iron-free Fenton systems.
4. Hybrid methods are not economically viable techniques to degrade large quantum of effluent disposed by the industries. Hence it is advisable to use these methods as pretreatment to reduce the toxicity to a certain level beyond which biological treatment can be employed.
5. Drawbacks associated with the use of Fenton and Fenton- based oxidation are the safety hazards associated with using H2O2 and the need to firstly reduce the pH, followed by a subsequent neutralization.
6. Chelating agents stabilize iron species in the solution and prevent sludge production at pH values higher than 4.0 and increase Fenton degradation efficiency through several routes.
Major attention should be devoted in the future on the identification of reaction intermediates, development of rate expressions based on established reaction mechanisms, identification of scale-up parameters and criteria for cost effectiveness. These studies should aim at addressing different challenges to overcome pH-dependency of the reaction and its future industrial applications by using solar energy which can minimize relevant energy cost.
For more articles in Open Access Journal of Environmental Sciences & Natural Resources please click on: https://juniperpublishers.com/ijesnr/
To know more about Open Access Journals Publishers
To read more…Fulltext please click on: https://juniperpublishers.com/ijesnr/IJESNR.MS.ID.555594.php




Comments
Post a Comment