The Ultrasonic of Drug Removal Using Catalysts from Aqueous Solutions - Juniper Publishers
Juniper Publishers- Open Access Journal of Environmental Sciences & Natural Resources
The Ultrasonic of Drug Removal Using Catalysts from Aqueous Solutions
Authored by Mohammad Hossein Sayadi
Abstract
The presence of drug substances in water is a serious environmental problem because many of them are poisonous and may accumulate in organisms and cause illness. The purpose of this study was to review the ultrasonic of drug removal using catalysts from aqueous solutions. Based on the results, optimal conditions for removing ibuprofen at 500 kHz frequency of ultrasonic apparatus were under acidic conditions and reaction time of 90 minutes, which was removed at 99.6%. While at low removal of ibuprofen in the frequencies of 862 kHz for 240 minutes, was 40%. The results of various studies showed that the ultrasonic process alone could not be used as an effective way to remove these compounds. Adding H2O2 and TIO2 catalysts greatly increased the removal process. Therefore, it can be concluded that this combination process is a beneficial way to remove the drug pollution in water and wastewater.
Keywords: Ultrasonic; Environmental pollution; pharmaceutical pollution
Introduction
Medications are a very important and integral part of today’s modern life and are used to treat human and animal illnesses. The presence of medications in the environment is one of the most important issues in the world today. Recently, only a few advanced countries have begun to study the negative effects of these pollutants on the environment [1]. These materials enter the environment in various ways, such as wastewater from pharmaceutical industries, hospitals and human and animal waste [2]. Drugs are detected in surface water, underground, wastewater and even in drinking water in amounts of nanograms to micrograms per liter. Various methods have been used such as active carbon adsorption, reverse osmosis, air buckling and biological methods to remove these drug compounds, but these methods do not eliminate the contaminants but transfer them from one phase to another Slowly [3,4].
Advanced oxidation
Advanced Oxidation Processes (AOPS: Advance Oxidation) are a good way to decompose pharmaceutical compounds, and so far, various processes such as photo electro co catalysis, [5,6] application of photo catalytic processes [7-9]. Photo phenton has been used to remove drugs from the aquatic environment [10]. These processes work on the basis of the production of hydroxyl radicals, which these radicals are almost capable of oxidizing all organic compounds, including these processes, the sono catalyst system. [10,11]. The sono chemical system, coupled with the use of the catalyst, is capable of oxidizing the drug and converting it to harmless or less harmful products for microorganisms [3]. Sono chemical reactions result from high-intensity sound radiation from liquids at frequencies producing cavitation (normally in the range of 1000 - 20 KHz). Therefore, cavitation as an ultrasound energy concentrator is introduced into the micro reactor, which acts simultaneously with the simultaneous release of reactive radicals to any server reactor as a hot spot. Ultrasound waves increase chemical and physical changes in a fluid environment through production and, consequently, the degradation of cavitation bubbles. These bubbles form over a period of a number of cycles and grow to equilibrate to a certain frequency. The destiny of these bubbles is that they collapse in the next intensive (compressed) periods to produce the energy required for chemical and mechanical effects (Michael, 2013). In addition, water sonolysis produces H2O2, which, coupled with the presence of a catalyst and the presence of iron ions, usually increases the decomposition of organic matter [12,13].
The main mechanism of destruction
The two main mechanisms for the destruction of pollutants during the sonolysical bubble are the pyrolysis reaction in cavitation and the other radical reactions by radical water, hydroxyl and hydrogen generated through sonolysis. The two mechanisms are generally as follows: [14]
Titanium dioxide catalyst
Between the various catalysts that can be used as a catalyst in this process, due to high catalytic activity, non-toxicity, stability in aqueous solutions and relatively few costs are commonplace [5]. The sono chemical process with the use of catalysts is relatively new to the process of water purification and AOPs of wastewater processes. Due to its high decomposition power, it can be used as an effective method for decomposition and degradation, such as those that have low biodegradability, such as ibuprofen [12].
Hydrogen peroxide catalyst
One of the strongest (H2O2) hydrogen peroxide is known and stronger oxidants than chlorine, chlorine dioxide, and potassium permanganate. The molecular structure of this material consists of an oxygen bridge, which connects each of these oxygen atoms to a hydrogen atom. This substance can react directly or indirectly with organic matter in water. Hydrogen peroxide acts in the oxidation-reduction reaction and acts as an oxidizing or reducing agent. In indirect reactions, the oxidizing activity is related to the free radicals produced by the decomposition of hydrogen peroxide. Previous studies have shown that the greater the amount of oxidation of substances, due to the fact that the result of a combined system is higher than OH the number of radicals of a separate ultrasonic system, therefore, the combined oxidation velocity will be higher than that of the separate ultrasonic system [15,16].
Removal of drug by ultrasonic process
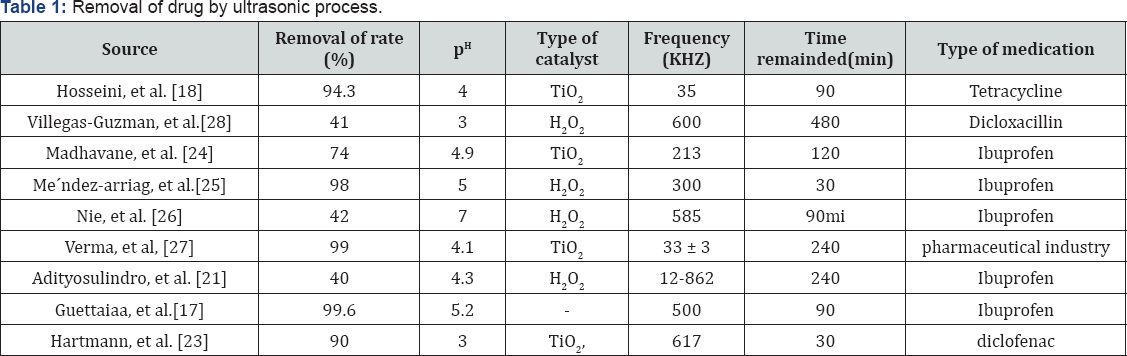
[17] In 2017 examined the sono chemical and photochemical removal of ibuprofen in aqueous solution and concluded that a combination of photochemical and ultrasonic purification resulted in a significant increase in the rate of IBP degradation and the best pH in this study was 5.2 [18] examined the efficacy of removing antibiotic tetracycline from aqueous solutions using a ultrasonic process and evaluating the effect of effective parameters on the process, The results of this study showed that the synolysis process alone cannot be used as an effective way to remove tetracycline antibiotics from aqueous solutions; However, the addition of a TiO2 catalyst improves the removal efficiency considerably, and therefore, this compound process can be used as a useful way to remove this biodegradable pollutant from water and sewage (Table 1)[19].
Examined the destruction of the chemical synthetic cephalexin in aqueous solution and reported that the ultrasonic process is a successful purification method for removing and improving the digestibility of cephalexin in the wastewater. Ultrasonic can effectively decompose cefalexin in aqueous solutions, and its degree of degradation depends on operating conditions, such as ultrasound power and pH value of the environment [20] investigated the ultrasonic degradation of tetracycline by sulfate radicals and reported that the removal efficiency was strongly influenced by the initial concentrations of tetracycline, pH, ultrasound strength, per sulfate concentration, temperature, and ion concentration. The destruction of tetracycline in silver and per sulfate was in the first degree kinetic son chemical process [21-24]. The study has shown that ultrasonic-based chemical oxidation is an effective and applicable technology for the destruction of organic compounds such as antibiotic tetracycline in aqueous solution [24-28].
Conclusion
Several studies have been carried out on the removal of pollutants from water and sewage, which shows that among the methods studied, advanced oxidation techniques based on radical hydroxyl production have the ability to remove and mineralize the drugs. In this review study, the process of drug removal using the ultrasonic process was studied. The results of this study showed that the ultrasonic process alone and individually due to its low efficiency does not require, a long time and has a high energy content and is not usable on large scale, and cannot be used as an effective way to remove drugs from water solutions , but the addition of TiO2 and H2O2 catalyst greatly improves the removal efficiency, and therefore This combined process was used as a useful way to remove these highly degradable biodegradable pollutants from water and wastewater. Therefore, it can be pointed out that this method can be used as an effective way to remove drugs and other similar pollutants.
For more articles in Open Access Journal of Environmental Sciences & Natural Resources | Juniper Publishers please click on: https://juniperpublishers.com/ijesnr/index.php




Comments
Post a Comment