Optimized Conditions for Bioleaching using Yeasts from Municipal Solid Wastes to produce Safe Compost or Fertiliser - Juniper Publishers
Juniper Publishers- Open Access Journal of Environmental Sciences & Natural Resources
Optimized Conditions for Bioleaching using Yeasts from Municipal Solid Wastes to produce Safe Compost or Fertiliser
Authored by Gregory A Tucker
Abstract
Bioleaching can be considering a simple and effective technology for removing metals from waste which is rich in heavy metals. Bioleaching of these metals by microorganisms offers an economical and eco-friendly approach. In this study Saccharomyces cerevisiae NCYC2592, was used to remove metals from municipal solid waste (MSW). The tolerance of S. cerevisiae against the metals was determined using phenotypic microarrays using artificial hydrolysate containing various metals. Parameters such as one and two way methods or process, yeast strains, pH and mediums were studied and optimized. The results revealed that two-way methods and S. stipitis strain among five strains, using artificial media correlated with better performance at pH 5.5, while at pH 4 Correlated with sorghum media which showed lowest retained yield %.
Keywords: Yeast; MSW; Heavy metals; Biolog; Artificial; Clay; Sorghum Medium
Introduction
The disposal method for municipal solid waste (MSW) is a major concern in most of urban areas, generally MSW derived from households, fruit and vegetable markets, canteens, hotels and juice centers, etc., are rich in organic matter and can be used for various bioenergy sources. Furthermore, using fossil fuels is one of the primary causes of global warming and acid rain, which affects the weather conditions, vegetation, and aquatic ecosystem. Nowadays to overcome these issues the development of non-polluting and renewable energy sources is required [1]. There are many alternative energy sources such as wind, solar and wave power. While, applying biomass can be considered as a sustainable energy source [2] that can be utilized to reduce the impact of energy production on the global environment. There are many types of biomass sources that can be converted to energy and each biomass feedstock has to be harvested/collected, transported and possibly stored, before being processed into a form suitable for the chosen energy conversion technology [3]. The selected biomass can be converted into the valuable bioenergy forms. However, there are many factors can influence the choice of conversion process such as the type and quantity of biomass feedstock; the desired form of the energy, i.e. end-use requirements; environmental standards; economic conditions; and project specific factors. [3], Biomass is the only renewable source that can be converted into convenient fuels through different conversion processes also, can be converted into three main products: two related to energy - power/heat generation and transportation fuels - and one as a chemical feedstock [4,5]. Nowadays due to the industrial expansion, large quantities of industrial wastes are accumulating in our environment and disposal can be difficult, in particular, wastes can contain heavy metals at high concentrations [6]. For this reason the wastes have to pass through special treatment to reduce the amount of heavy metals (copper, zinc, lead, chromium, etc.)[7]. Wastes contain metals in a various levels can be discharged directly or indirectly in to the environment, which can cause a serious environmental pollution, and threatened bio life [8]. These metals classified as the following three categories: toxic metals (such as Hg, Cr, Pb, Zn, Cu, Ni, Cd, As, Co, Sn, etc.), precious metals (such as Pd, Pt, Ag, Au, Ru etc.) and radionuclides (such as U, Th, Ra, Am, etc.), whose specific weight is usually more than 5.0 g/ cm3 [8].Toxic metals can effect on the environment in a different ways : (1) the toxicity can stay for a long time in nature; (2) heavy metals can be transformed from relevant low toxic species into more toxic forms such as mercury (3) also can effect human health by the bioaccumulation and bio augmentation of heavy metal by food chain finally; (4) metals can only be transformed and changed in valence and species, but cannot be degraded by any methods including bio treatment; (5) the toxicity of heavy metals occurs even in low concentration of about 1.0-10 mg/L. Some metal ions, such as Hg and Cd, are very toxic even in lower concentration of 0.001-0.1 mg/L [9,10].
Removing heavy metals from aqueous solution can be carried out using many methods such as chemical precipitation, ion exchange, electrochemical treatment membrane technologies, adsorption on activated carbon etc [11]. However chemical and electrochemical method are inefficient, due to the production of large amounts of sludge, which are difficult to treat or are very expensive which make them not suitable for large scale applications [12,13]. Heavy metal removal by biosorption has been extensively investigated during the last several decades [14]. Some reviews have been published focusing on different aspects of heavy metal biosorption [15-18]. From these reviews, we can see that the research on biosorption is focused on the following three major fields. First, the biosorbents [19]; second, the mechanism of biosorption; third, large-scale implementation. The biosorption process is currently at the stage of laboratory-scale study and converting into a large- scale process has proven very difficult [20]. An alternative method for the previous method is microbial leaching [21]. Bio hydro metallurgical (bioremediation) is considered as a green technology, with low cost, low energy requirement. They can effectively sequester dissolved metal ions out of dilute complex solutions with high efficiency and quickly [22]. There are three groups of microorganisms has been used for bioleaching these are autotrophic bacteria (e.g. Thiobacillispp.), heterotrophic bacteria (e.g. Pseudomonaspp., Bacillusspp.) and heterotrophic fungi (e.g. Aspergillus spp.,PeniciIIium spp.) [23]. Furthermore, yeast and algae can remove heavy metals from aquase solution in substantial quantities [24]. The most common yeast used in many studies is Saccharomyces cerevisiae and is used extensively in the food and beverage industry. S. cerevisiae has been shown to be a Midcore biosorbent, and has a unique characteristics comparing with other microorganisms with regards to removal of metal from a solution or solid phase [25]. Advantages of S. cerevisiae as a biosorbents for metal biosorption include: ease of cultivation, can be obtain from various food and beverage industries, yield of biomass is high, and is considered a safe organism [26]. The objective of this study is to investigate resistance of S. cerevisiae to the presence of heavy metal sand to determine the efficacy of using bioleaching on MSW using a one- step or two-step process. The physical and chemical especially heavy metals characterization of the MSW was initially carried out pre-bioleached using S. cerevisiae and other five yeast strains. The different constituents of the artificial medium were analyzed and comparing it with non-artificial medium using pH 5.5 and 4. The metal extraction yields in bioleaching were measured in MSW biomass to produce safe fertilizer. MSW physical and chemical composition and elements analysis were analyzed and represent by [27].
Material and Methods
Microorganisms
Yeast strains -Saccharomyces cerevisiae (NCYC2592); Kluyveromycesmarxianus, Schefforsomyces stipitis, Candida succiphilia(NCYC1403), Pichia gulliermondii(NCYC 443), and Candida shehatae(NCYC2389). All strains were obtained from the National Collection of Yeast Cultures (www.ncyc.co.uk). Yeast strain and growth conditions, these strain were maintained on agar containing 10g/L yeast extract, 20g/L peptone, 20g/L glucose, and 20g/L agar (YPD agar) and grown on 10g/L yeast extract, 20g/L peptone, and 20g/L glucose.
Artificial Media used to Establish the Tolerance to Metals in Yeast
Stock solutions (0.1-4mM) respectively, for Mn(II) (MnSO4),Fe(II) (FeSO4), Pb(II) (Pb(NO3)2), Zn (ZnSO4), Al Al2(SO4)3 , and Cu2+ (II) , CuSO4 were prepared by dissolving in reverse osmosis (RO) water. Chemicals were provided at analytical grade by Sigma-Aldrich. Metal solutions between 0.1-3mMwere prepared from stock solutions for each metal. The pH was maintained at 5.5 by adding 3M NaOH. The liquid hydrolysates were taken forward for assessment of yeast metabolic output using phenotypic microarrays (Biolog, CA, US). Assessment of metabolic activity, preparation and assays under control conditions (6% glucose, 0.67%YNB and 0.2 μL dye D (Biolog, Hayward, CA, USA)) were according to [28]. Metabolic output using hydrolysates was determined by adding 29.8 μL hydrolysate with 0.2μL dye D (Biolog, Hayward, CA, USA) prior to addition to plates pH was established at pH 4.8-5. The second part is strain preparation for inoculation into the PM assay plates as follows. Cells were grown in YPD broth at 25oC and were inoculated into sterile water in 20 x 100mm test tubes and adjusted to a transmittance of 62 % (~5x106 cells/mL) using a Bio log turbid meter (Biolog), to prepare cell suspension. Cells were mixed with IFY buffer ™ (Biolog) (1:5) and90 μL of cell suspension inoculated into each well in a Biolog 96-well plate. The prepared plates were then placed in the Omni Log reader and incubated for 96hr at 30oC under aerobic conditions. The principle of Omni Log reader is that the PM plates are photographed at 15min intervals, and the pixel density in each well converted to a signal value reflecting dye conversion. Dye reduction reflects metabolic activity of the cells which has been defined here as the redox signal intensity. After completion of the run, the signal data was compiled and exported from the Bio log software using Microsoft® Excel. In all cases, a minimum of three replicate PM assay runs were conducted, and the mean signal values are presented.
Media used for Bioleaching Artificial Medium
Yeast Artificial medium- the yeast medium includes two artificial mediums;Medium1 contained (g/L): 120 glucose, 10 yeast extract, 20 peptone; Medium 2 was based on the study by [29] the medium contained (g/L) 20 glucose, KH2PO4, 2.72; K2HPO4, 5.22; (NH4)2SO4, 2.0; MgSO4 7H2O, 0.5; FeSO4•7H2•, 0.0022; ZnSO4•7H2C), 0.004, MnSO4 4H2O, 0.004; CuSO4•5H2O, 0.004 and altered using to either pH 5.5 or pH 4). The other mediums used in the study have been described previously [30].
Bioleaching Process
To study the biosorption rate cultures were grown at 30oC, 180 rpm, pH 5.5 in a prepared liquid artificial mineral and yeast common yeast liquid medium as mentioned previously. The constitution of basal medium was based on the study by [31,32]. For MSW bioleaching cultures where grown as mentioned previously in various medium and to get optimum bioleaching process many optimizing conditions has been taken into the consideration using 2% MSW substrate. After 15 days, samples were centrifuged at 10,000g for 20mins, the liquid fraction was decanted and the solid fraction was washed 3 times using RO water and dried at 70oC, weight loss (%) in the solid fraction was determined and presence of heavy metals in both fractions determined. The samples were filtered through 0.45μmmember filters before the following analysis elements were selected in this study phosphorus (P2+), Potassium (K+), Chromium (Cr6+), Copper (Cu2+), Zinc (Zn2+), Cadmium (Cd2+), and Lead (Pb2+), finally the yield was determined in the solid fraction. The metal extraction efficiency was calculated as the % recovery in the MSW.
Analytical Methods
Heavy metals-Dried solid fraction was digested using concentrated nitric acid the liquid fraction taken forward to measure heavy metals released. Heavy metals analysis was as described in by [30].
Results
MSW physical and chemical composition and analysis were analyzed and represent by [27].
Effects of Heavy Metals on Yeast Metabolism
Yeast can be used as agents for bioleaching of heavy metals from MSW; however, tolerance to heavy metals in yeast is not yet fully understood. The effect of heavy metals on yeast metabolism was thus determined using a phenotypic microarray assay. It was determined that S. cerevisiae NCYC2592 displayed tolerance to Mn2+, Zn2+or Al3+ up to 4mM (Figure 1). However, presence of other metals such as Pb2+, Fe2+ and Cu2+ at 4mM reduced metabolic output when comparing with control conditions (Figure 1). Presence of Cu2+ at 0.1-0.5mM actually improved metabolic output when compared with control conditions. These results suggest that the yeast could tolerate the presence of Mn2+, Zn2+ and Al3+ at concentrations relevant to those found in MSW. Bioleaching with S. cerevisiae NCYC2592 using either a one or two way method. Yeast were incubated with MSW as described in the methods using the two media and the two bioleaching methods and the impact on bioleaching monitored as for the filamentous fungi. The constitution of basal medium was based on the study. Comparing Retained yield (%) it was shown that P using a one way method had the highest yield when compared with other elements in all conditions, the results shown from total metals average that lowest retained yield % was by using medium one and two way method. The results are shown in Figure 1. The 8 elements retained from MSW were taken forward for ANOVA testing and results revealed that there was no significant difference between methods and media in terms of retained yield % of elements (Table 1). These results suggest that the two way method and medium 1 was the best combination comparing with the control. Assays using medium one and two way method showed the lowest retaining yield when compared with water. Further experiments were carried out to increase the retained % yield (Figure 2).


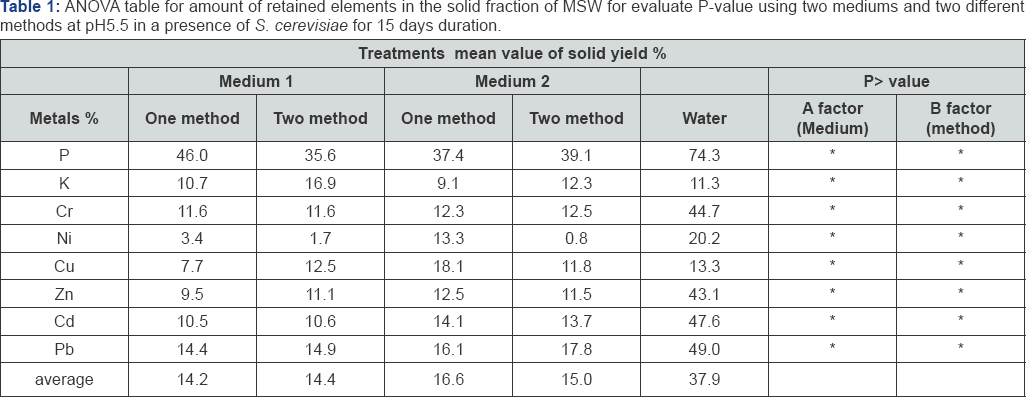
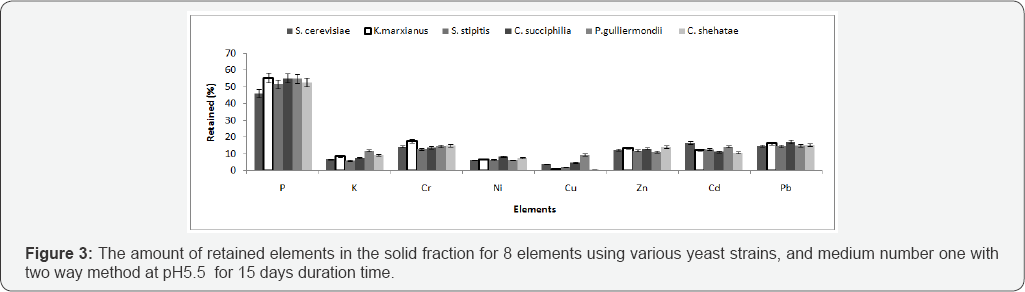
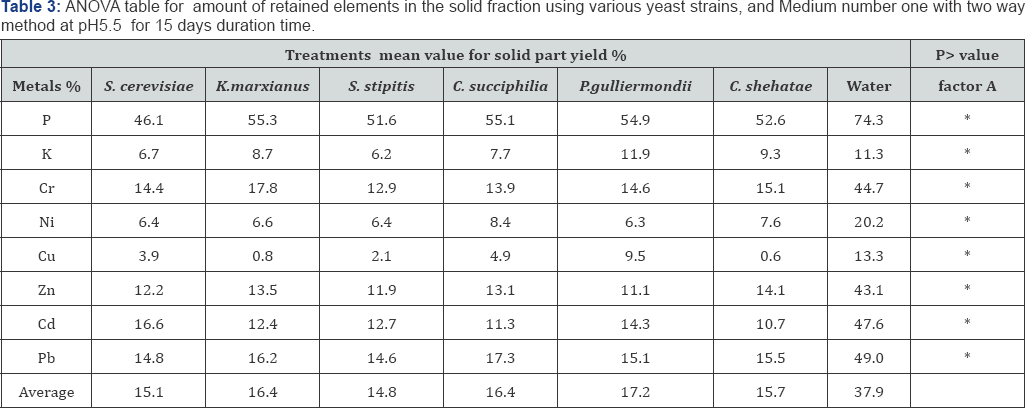
Comparing yeast strains for bioleaching of MSW From the previous experiments the two way method and medium 1 was found to be the best combination. Different yeast strains were compared including S. cerevisiae for their capacity to remove metals from artificial mediums (Figure 3). The results revealed that the retained %yield was lowest (best condition) using S. stipitis when compared with other strains. In terms of elements retained from the solid fraction the highest for all strains were P3+and Se2- (Figure 3). ANOVA analysis on the 8 elements retained in the MSW residue revealed no significant difference between yeast strains; however, all the yeasts had a significantly improved retained % yield when compared with the control. From this result S. stipitis and S. cerevisiae were further studied to optimize bioleaching conditions of MSW using different media and pH.
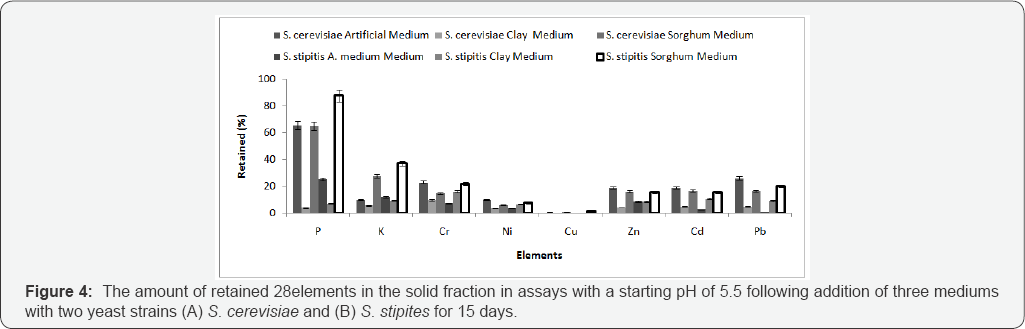
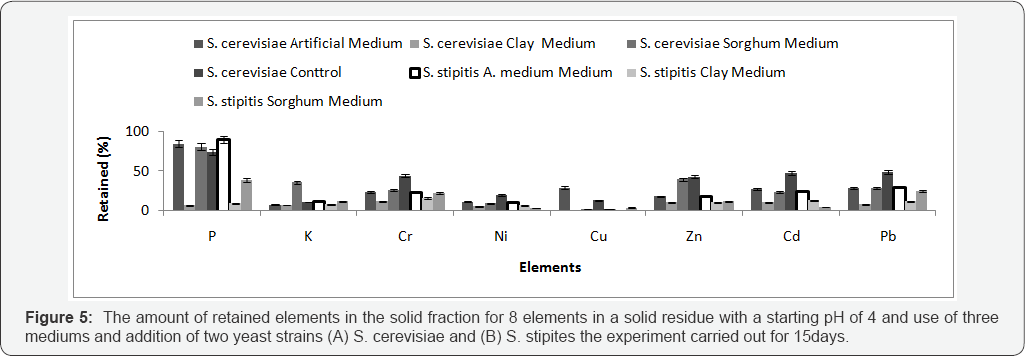
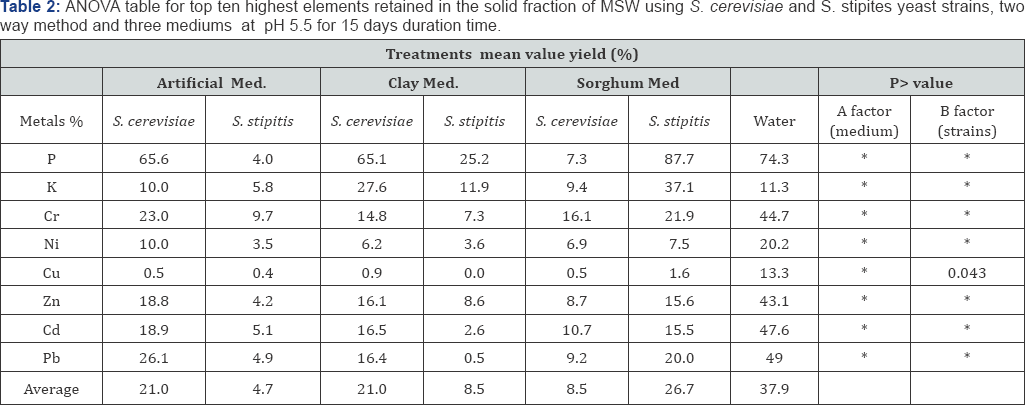
S. cerevisiae NCYC2592 and S. stipitis NCYC 1541 were inoculated in medium 1 and non-artificial media prepared at either pH 5.5 or 4 and the bioleaching experiment carried out for 15 days (Table 2). Comparing retained % yield in the solid residue, it was observed that presence of sorghum in the medium (S. cerevisiae) and artificial medium (S. stipitis) correlated with an improved removal of metals from MSW (Figure 4). ANOVA analysis for the 8 retained elements, determined that use of artificial medium and S. stipitis as a strain had the highest ability in terms of removing elements from MSW (Table 3). Analysis revealed that for some elements, Cd and Cu concentrations of those elements still exceeded safe disposal limits for compost use. Further assays were carried out to look at the effect of pH on the bioleaching of these elements. Assays with a starting pH of 4 revealed that P3-, Rb+ and U4+, where the highest elements with the lowest retaining % yield (best performance) recorded using sorghum media S. stipitis (Figure 5). ANOVA analysis revealed that by using sorghum medium (S. stipitis) at a starting pH of 4 showed lowest retained % yield (best condition) when compared with other conditions. ANOVA analysis revealed that there was a significant difference between mediums, use of sorghum as a medium and addition of S. stipitis correlated with better retained yield (Tables 4 & 5). Comparing pH 5.5 with pH 4 the results showed that pH 5.5 was better for retaining yield % from the solid fraction of MSW when compared with pH 4. The best retaining yield was 13.8% using artificial medium pH5.5 using S. stipitis while comparing the lowest retained yield % with the washed MSW, the optimum condition showed a significant improvement comparing with washed MSW sample (the retained yield % using water was 33.25) , as shown in (Figure 6).


Discussion
This study revealed that S. cerevisiae displayed tolerance to various metals, as has been reported previously by [33] and this has highlighted the potential for the use of S. cerevisiae as an agent for removing these metals from MSW. In this study, concentrations of Cs+ and Ca2+ in MSW ranged from 0.0032- 517.6mg/L respectively. The study also revealed that presence of Cu2+, Fe2+ and Pb2+ at high concentrations correlated with a reduction in yeast metabolic rates. In addition, an increase in number of metals decreased bioleaching process due to number of metal ions competing for the same binding sites available on the surface of the biomass [34,35]. Single and two-step processes have been applied for bioleaching using fungi; thus in this study the two methods were also applied for yeast bioleaching. The results revealed that use of a two way method was better than the one way method. There's no published work on the optimisation of bioleaching processes using yeast. One advantage of the two stage method is that the bioprocess and the chemical processes are independent and this makes it possible to optimise each process independently to maximise productivity [36].
Use of a two-step way process permits enhancement during each stage, with the biological oxidation stage being fast and effective [37]. In addition, metals from fly ash were bioleached more rapidly using a two-step approach when compared to a one-step approach, resulting in earlier formation of calcium oxalate hydrate, which leads to more rapid decrease in pH occurring in a two-step bioleaching methods since organic acids were already present in the medium prior to the addition of fly ash. However, the addition of fly ash after fungal germination in a two-step bioleaching effectively reduced the toxic effects on the fungal growth, and accelerated the bioleaching process [38,39]. With regards to optimizing the medium for bioleaching, use of medium one correlated with better performance when comparing with use of an artificial medium, first medium has been shown to be a natural yeast growth medium, while in the second medium some minerals have been added which caused an increase in the concentrations of metals or elements and led to less bio-leaching of heavy metals [40].
Results comparing different yeast strains revealed that S. stipitis gave the best performance in terms of leaching metals, this result agreed with published findings, which found that some yeast strains (vacuole efficient mutants) were better than wild type S. cerevisiae, in terms of leaching some metals [41]. Studies have revealed that Candida sp. and K. marxianus were more efficient than S. cerevisiae or S. pombe for heavy metal resistance and copper (II) bioaccumulation at higher copper(II) concentrations [42,43]. While, this study's findings did not correlate with the observation that that S. cerevisiae produces a dark melanin pigment and extracellular polymers whose presence as part of a defense mechanism can reduce the toxic effect of heavy metals.
Experiments comparing initial pH and media for bioleaching of heavy metals from MSW determined that starting the bioleaching (retained % yield) at pH 5.5 was better than at a pH of4. Bioleaching medium pH is consider an important environmental factor which effects yeast performance, site dissociation of the biomass’ surface, effects on the solution chemistry of the heavy metals, [40,41]. The improved performance at pH 5.5 could be correlated with higher metal solubility and agreed with a study which revealed that by increasing pH the bio absorbitive capacity of metals also increased [44]. In addition, high metal bio-sorption was obtained with an initial metal concentration ranging from between 50-100mg/L at 30°C temperature and pH 5.0. Generally, for different bio-sorption systems for metal ions, optimal pH differs depending on the metal e.g. the optimal pH value bio-sorption by S. cerevisiae for copper is 5-9, and 4-5 for uranium, and in general the optimum bioleaching pH is above pH 5.
However, when pH increases over optimal level, bio-sorption capacity has been shown to decrease which may be due to the fact that the efficiency of the proton at the binding site of yeast is much smaller than that of the metal ion comparing with low pH so the electrostatic attraction to negatively charged functional groups may be one of the specific bio-sorption mechanisms at higher pH's [43]. It has been observed that cell wall ligands were closely associated with the hydronium ions [H3O+] and restricted the approach of metal cations as a result of the repulsive force at low pH [42]. At pH 5 divalent positive ions could interact with negatively charged groups within the biomass. On the other hand, the outer layer of the cell wall of S. cerevisiae consists of coat protein, which can cause a charge through dissociation of ionisable side groups on amino acids [45] , also inorganic ions such as chloride, may form complexes with some metal ions and thereby affect the sorption process [46] . Using higher pH's correlates with higher consumption of hydroxides and precipitation of other metals [47]. Generally speaking, a low pH value is favorable for bio-sorption.
Anions (carbonates, chlorides, fluorides, phosphates and sulphates) in solution can complex metal ions and thus reduce or inhibit their adsorption by S. cerevisiae [48]. Redox potential can also affect the speciation of a given element; for example, chromium exists as Cr(VI) or Cr(III) according to the EH of the solution. Presence in solution of other cations besides the metals of interest can reduce heavy metals accumulation by biomass, by competition for binding sites on yeast cell wall [49,50]. With regards to the selection of the best medium from the current study, it is suggested revealed that presence of artificial media and S. stipitis at pH 5.5 recorded highest retained % yields because of addition of glucose and other trace elements could enhance cells growth in a system, and facilitate metal biosorption. There are some studies focused on effects of glucose on bioleaching rates which have revealed that pre-treatment of the yeast cells with glucose increased the amount of Cu2+, Cr6+, Cd2+, Ni2+ and Zn2+ removed from electroplating effluent by living cells of S. cerevisiae [51].
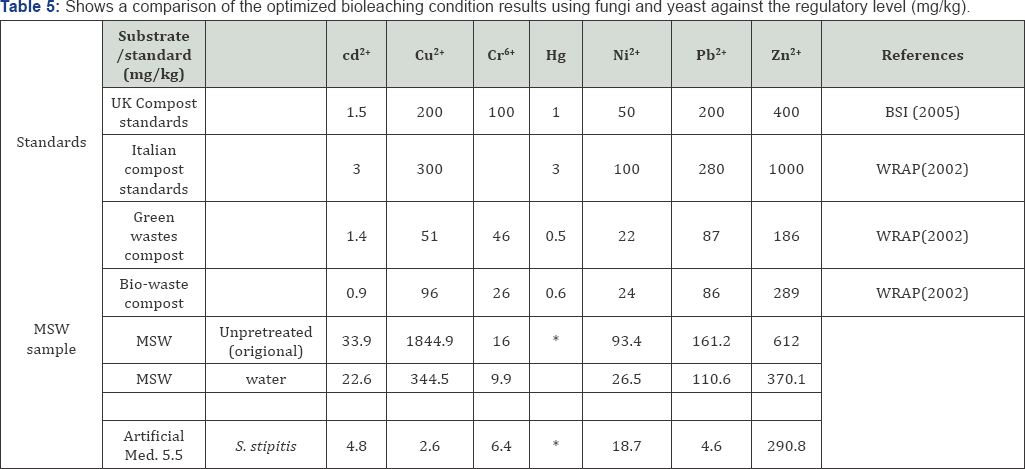
Direct addition glucose in to the yeast effluent mixed solution had no effects on the amount of bioleaching and this could be a possible reason for Sorghum medium having more effects than artificial medium in terms of leaching metals from MSW. Furthermore, it has been revealed that addition of cysteine, glucose, ammonium sulphate, phosphate and ammonium chloride in the fermentation media increases uptake of Cr (VI) [52]. Generally different nutrients clearly led to different functional groups in the corresponding cell surface. Toxicity characteristic fungal and yeast bioleaching process, are shown in Table 5 showed that following bioleaching using yeast the applicable environmental standards for presence of metals was almost achieved, the table shows MSW before and after the bioleaching process were compared with the identification standard for hazardous wastes identification for extraction procedure toxicity (State Environmental Protection Administration, 1996) and the standard for pollution control on the security landfill site for hazardous wastes (State Environmental Protection Administration, 2001) set by State Environmental Protection Administration, China.
Conclusion
The objective of this study was to examine how growth of microbial such yeast could correlate with removal of heavy metals from MSW. Bioleaching with yeast has been studied and it was determined that using a two-way methods and S. stipitis strain gave the best results then to increase the yield two ranges of pH has been applied pH 5.5 and 4, using sorghum and clay mediums. Addition of clay into the medium correlated with better performance at pH 5.5 and pH 4 and was characterized by the low presence of heavy metals in the liquid fraction as clay particles absorb the metals.
For more articles in Juniper Publishers | Open Access Journal of Environmental Sciences & Natural Resources please click on: https://juniperpublishers.com/ijesnr/index.php




Comments
Post a Comment