Systemic Plant Pathogen Botrytis Cinerea Influences f the Feeding and Mating on Myzus Persicae by Parasitoid Aphidius Ervi - Juniper Publishers
Systemic Plant Pathogen Botrytis Cinerea Influences f the Feeding and Mating on Myzus Persicae by Parasitoid Aphidius Ervi
Authored by Yahaya SM
Abstract
Female parasitoids assess host quality and make a decision whether to lay egg or not. Here we investigated the relationship between the green peach potato aphid Myzus persicae Sulzer (Hemiptera: Aphididae) and its common parasitoid, Aphidius ervi Vierek (Hymenoptera: Braconidae), to determine whether the relationship is influence by infection of the aphids host by B. cinerea. In hymentopteran parasitoids which are haplo- diploid, the females may selectively lay female or male eggs by controlling fertilization depending on whether the host is infected with B. Cinerea or not. Apart from infection of B. cinerea many other factors may influence the female's decision to fertilize and create biased offspring sex ratios: host quality, environment and local mate competition. It is clear that here attention was directed on parasitoids assessing host quality, especially the host size. While there was a significant effect of host plant infection on aphid size (measured as hind tibia length), and a resulting significant effect of fungal infection on the size of emerging parasitoids, there was no evidence of reduced survival or attack rates of parasitoids reared on aphids feeding on infected plants, and there was no difference in the sex ratio of parasitoids emerging from aphids reared on infected and control plants. Offspring sex is therefore not absolute; it mainly depends on relative host variability and the parasitoid's previous experience. Therefore, future research on sex allocation should consider factors that do not involve females manipulating fertilization that can create biased sex ratios.
Keywords: Aphedius ervi; cinerea; Myzus persicae; Interaction. Choice
Introduction
Herbivorous insect aphids (Hemiptera, Aphididae) are widely distributed small plant-sucking insects colonizing over 3000 species of herbaceous plants and shrubs and many are found on trees [1]. Many aphids specialize on a few species of plants and plant selection is normally through the use of chemical cues [2]. Aphids mainly occur in colonies and their growth dynamics often results in high population sizes, [3,4]. Therefore, aphids are important agricultural pests, which in addition to causing direct damage by feeding on crops, cause indirect damage by acting as vectors of viruses which may cause significant diseases of their host plants [5]. The aphid Myzus persicae (Sulzer) is a serious pest of many important agricultural crops such as peaches, potatoes, sugar beets, and tobacco, and various ornamental crops grown in landscapes and in glasshouses [6]. High population of M. persicae on crop plant can causes injury by removing large volumes of sap from the plants and depleting them of the needed nutrients. In addition they may also cause indirect injury by the production of sugary honeydew which makes the leaves susceptible to microbial attack, which then reduces leaf quality [6]. Many aphids are widely attacked by a range of natural enemies such as hymenopteran parasitoids (e.g.Aphidius species such as Aphidius ervi, A. matricariae, A. ervi and A. abdominals), and a wide range of arthropod predators [5,7]. High aphid numbers on a plant serves as a resource for their natural enemies. Many researchers have investigated the effects of natural enemies for keeping aphid population sizes below economic injury levels [5,8-10]. Also aphids may indirectly interact with systemic pathogens such as Botrytis cinerea on the host plant and both aphids and pathogens may induces the host plant to release various chemicals for defence, serving as cues for foraging natural enemies [11,12].
The parasitoid Aphidius ervi belong to the sub family Aphidiidae (Hymenoptera: Braconidae) the females of Aphidius ervi lay their eggs inside the aphids. Aphidius ervi being Koinobiont endo parasitoid as the eggs hatch the larvae feed off the living host, killing it by the fourth and last instar. The larvae formed a protective cocoon inside the exoskeleton of the aphid before reaching the adult stage. The unfertilized eggs develop into males, while fertilized eggs develops into females (haplodiploid) which later seek for a new hosts after emergence [13-18]. Female hymenopteran parasitoids select hosts which provide the best resources for their offspring [19,20]. They select hosts, and control the sex of the offspring by influencing fertilization, evidence support that female hymenopteran manipulates the release of sperm from the spermatheca while eggs pass through her genital tract, the unfertilized eggs develop into males while fertilized eggs develop into females [21-26,18]. As fitness is positively correlated with size in female parasitoids, but not for males, female parasitoids may therefore, selectively oviposit male eggs in poorer quality host when given a choice [27]. This research is aimed at determining whether the presence of systemic pathogen B. cinerea infecting a model plant system [the lettuce, Lactuca sativa L. (Asteraceae: Compositae)] influences the interaction between an insect herbivore (the peach potato aphid Myzuspersicae) and a parasitoid widely used in aphid biocontrol, Aphidius ervi. Three hypotheses were tested. First, Myzus persicae feeding on infected lettuce plants would be smaller. Second, parasitoids emerging from aphid host feeding on infected plants would be smaller. Third, host plant infection would influence the relationship between aphid M. persicae and A. ervi, resulting in fewer offspring and a more biased sex ratio when parasitoids attacked aphids feeding on infected plants.
Material and Methods
Experimental plants
Lettuce seed (Tom Thumb) infected and uninfected by B. cinerea obtained by inoculating lettuce plants at the flower stage and resulting seeds tested by plating on Botrytis selective media (BSM) plates were the seed source used in this experiment. Lettuce seed were individually sown in 60 (30 infected, 30 uninfected) 15cm pots filled with a vermiculite growing medium in a controlled environment (18-200C, L: D 12-12, ambient humidity).
Infestation with Myzus persicae
Three weeks after germination all plants were infested with 20 adult M. persicae by placing them individually on the reverse side of the leaves using a small moist brush. Plants were then covered with a transparent plastic cup for 48 hours. All adult aphids were then removed leaving second instar nymphs in situ. While this prevents absolute control of experimental numbers it avoids the possibility of damage to the delicate nymphs during individual transfer. Aphid colonies were covered at all times by a vented plastic container, preventing escape of aphids or parasitoids.
Attack by Aphidius ervi
Aphidius ervi is another commercially available biocontrol agent, and experimental samples were obtained from Koppert UK (1967). The female A. ervi oviposit a single egg into aphid nymphs. The larva develops inside the body of aphid host until it kills the host. The experimental A. ervi were feed with adlibitum honey and maintained in vials at 5 OC in the laboratory Prior to use parasitoids were allowed to acclimatize to room temperature. Immediately after the adult aphids were removed from the plants, the nymphs were exposed to attack by five female Aphidius ervi. Twenty infested plants grown from infected and uninfected seeds were attacked with the aphid natural enemy while the aphids on the remaining ten infected and uninfected plants were allowed to serve as an unattacked control.
Attack rate
During the study a visual observation was carried out every day for the emergence of mummies. A week after aphid natural enemy attack the young aphids that became adult were counted and collected also the mummified aphid were counted and collected separately in to 200ml Eppendorf tubes and placed on a laboratory bench for one week to allow the emergence of the parasitoids.
Size of Myzus Persicae and Aphidius ervi
Myzus persicae, ten adult were haphazardly selected from all plants while all the resulting parasitoids from the mummies were used for the determination of hind tibia length. Each Myzus persicae and Aphidius ervi was placed in a drop of 100% ethanol on a glass slide covered with a cover slip and observed using a micrometric eye piece attached to a microscope (Nikon) at 50 x magnification and calibrated with graticule.
Statistical analysis
Data was analysed using Analysis of variance (ANOVA) using R [28-30]. Since the data conformed to the assumptions of normality data on hind tibia length was not transformed prior to analysis. However, data on attack rate and sex ratio was analysed using a generalised linear model using binomial errors [29].
Results
Size of aphid Myzus persicae
The aphid s reared on plants uninfected by B. cinerea were larger than those reared on infected plants (F1, 39 = 281.08 P < 0.001, Figure 1).
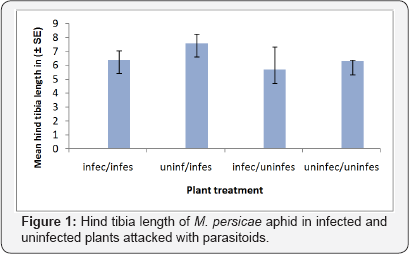
Size of parasitoid Aphidius ervi
The size of the Aphidius ervi was indirectly affected by Botrytis cinerea infection. The hind tibia length of Aphidius ervi that emerged from aphids reared on uninfected plants was significantly longer than parasitoids reared on aphids feeding on infected plants (F1, 79 = 70.13, P < 0.001). Sex of the Aphidius ervi was affected by the infection of B. cinerea as female Aphidius ervi were significantly larger than male Aphidius ervi when reared on both infected (Fa, 79 = 181.92 P < 0.001) and uninfected plants. However, the interaction between sex and infection was not significant on the hind tibia length (Figure 2, F1, 79 = 0.07 P =0.766).
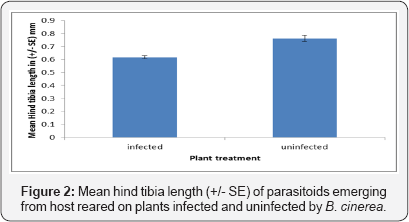
Sex ratio and Parasitism rate of Aphidius ervi
The rate of attack suffered by M. persicae reared on uninfected plants was higher than in infected plants (Figure 3, F1, 39 = 71.554 P = 0.0813). However, there were a greater number of aphids and parasitoids obtained from the uninfected plants. However, there was no significant effect of plant infection status on the sex ratio of emerging A. ervi.
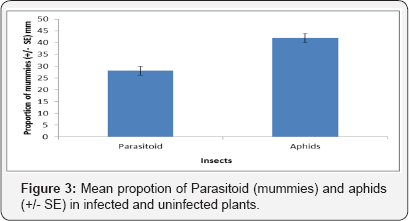
Discussion
High number of Myzus persicae and their parasitoids (Aphidius ervi) were reared on lettuce plants grown from seed uninfected with Botrytis cinerea. However, parasitoids attacked proportionally more aphids on infected plants, and both aphids and emerging parasitoids were significantly smaller when reared on infected plants. Similarly, there was no difference in parasitoids sex ratio, with 50:50 sex ratio found with parasitoids emerging from host reared on both infected and uninfected plants.
The study found that A. ervi emerging from small aphids, showed a significant male biased offspring sex ratio. This shift in emergence sex ratio may be the results of a significantly higher mortality of female progeny in small than large aphids. The female A. ervi often have more strict nutritional requirements compare to males and may therefore reach a larger size than males even in a hosts of equal size but the females takes longer time to complete their development, this is commonly reported in koinobionts were the female parasitoid have extended period of larval development on growing host which enable them to consume more resources than the male offspring [31-33]. Therefore the higher female mortality in small aphids normally occurs due to shortage of resource during early stages of female development in small aphids. Therefore the sex ratio determined by female choice at oviposition may be altered by mortality of the sexes. A biased sex ratio may result either from the female's control of fertilization at oviposition, or, may results, from the differential mortality of male and female offspring during early stages of development [19]. The results revealed that the aphid hosts are larger when reared on uninfected plants than infected ones; this was shown by the possession of longer hind tibia in aphid reared on uninfected plants. Body size in aphid host is an index of host quality where host quality is a linear function of size and the quality determined the ability of the foraging parasitoid to obtain required nutritional resources from the host [20,31]. Larger host are more nutritious and a good resource for the growth and development of offspring, and influence the survival and fecundity of the parasitoids, although larger aphids are more difficult to capture by the parasitoids yet our finding shows that female A. ervi oviposit more in uninfected host species because their offsprings have higher probability of survival [20,30,34].
The study showed that low number of parasitoid mummies was recorded in aphid reared on B. cinerea infected plants, which indicated the negative effects of B. cinerea on the tritrophic interactions in both preference and performance. Botrytis cinerea reduced the fitness of the aphids resulting in poor- quality host and low nutritious values with a high mortality rate. This can be explained by ability of plant pathogen B. cinerea to induce the production of secondary metabolites by the plant which may have toxic, antifeedant or aversive effects on the aphids. Therefore, the foraging parasitoid discriminates between infected and uninfected aphid host whether to feed, oviposit or to ignore, because host choice correlate with offspring performance which has a direct impact on the fitness. The parasitoid that feed on aphid reared on infected plants became infected. However, after oviposition not all the eggs will give rise to new off springs as egg laid in a poor-quality host are destined to die or give rise to inferior adults. Other negative effects of plant pathogen B. cinerea on the tritrophic interaction are indirectly influencing sex manipulation by the female parasitoid. Size or quality and mating of sibling are the main factors influencing the sex ratio which is dependent upon the presence or absence of plant pathogen. Female parasitoid tends to lay male eggs on a poor- quality host which developed to small adults while lays female eggs on a good-quality host which give rises to large adults. The reason for this is the success of male reproduction is less contingent on size as compare to female fecundity, therefore, male benefit less by being larger than female [19,14].
Quality of the plant directly and indirectly affected the interaction between aphids and parasitoids, and determined aphid size and biology of the parasitoids. In this study, uninfected plants serve as the better host for the aphid M. persicae by providing sufficient nutrient sources, which ensured large size of the aphids an indication of high plant quality [35]. High quality and fitter aphids serves as a good resource for the parasitoid feeding and oviposition however, the aphids reared on infected plants lacks required nutritious values the aphid becomes infected and less fit. For example, in comparism with the present study, Francis et al. [36] reported that M. persicae suitability as prey for A. bipunctata was compromised when the aphid was reared on Brassica species with rise levels of glucosinolates. Volatile secreted by herbivore -infested plants are attractive to parasitoids therefore mediate the interaction between aphids and their parasitoids. Therefore, the response of the parasitoids to the plant volatiles is proposed as an indirect plant defensive strategy reducing the number of aphids exposed to the plants [37-39]. The parasitoid shows some associative learning with a particular volatile blend with the presence of hosts after exposure in a host encounter. The associative learning enables parasitoids to cope with complexity of the environment by focusing on suitable cues.
In the present study, we found that in lettuce plant, there was an indirect interaction between B. cinerea and aphids, resulting in the decreased B. cinerea lesions and aphid biomass. Both B. cinerea and aphid M. persicae induced the plant to secret chemical's substances in a form of defense which serve as a cue for the aphids natural enemy parasitoids, a biocontrol which regulates the number of aphids. By reducing the number of insect herbivores, parasitoids reduce a considerable loss in crop yield [17,40]. This interaction helps in maintaining stable population dynamics in a long-lived ecosystem. The study has shown that infection by B. cinerea affects the interaction between the aphid M. persicae and their parasitoid A. ervi. The infected aphid host is less fit and lacks enough resource to support the optimum growth of A. ervi. In the present study, we measure fitness in terms of the number of offspring produced and the body size of the emerging offspring the fitness of the A. ervi is compromised where they feed on aphids reared on host plants infected with endophytic B. cinerea.
For more articles in Juniper Publishers | Open Access Journal of Environmental Sciences & Natural Resources please click on: https://juniperpublishers.com/ijesnr/index.php




Comments
Post a Comment